Riluzole Administration to Rats with Levodopa-Induced Dyskinesia Leads to Loss of DNA Methylation in Neuronal Genes
- PMID: 34207710
- PMCID: PMC8228416
- DOI: 10.3390/cells10061442
Riluzole Administration to Rats with Levodopa-Induced Dyskinesia Leads to Loss of DNA Methylation in Neuronal Genes
Abstract
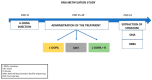
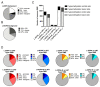
Dyskinesias are characterized by abnormal repetitive involuntary movements due to dysfunctional neuronal activity. Although levodopa-induced dyskinesia, characterized by tic-like abnormal involuntary movements, has no clinical treatment for Parkinson's disease patients, animal studies indicate that Riluzole, which interferes with glutamatergic neurotransmission, can improve the phenotype. The rat model of Levodopa-Induced Dyskinesia is a unilateral lesion with 6-hydroxydopamine in the medial forebrain bundle, followed by the repeated administration of levodopa. The molecular pathomechanism of Levodopa-Induced Dyskinesia is still not deciphered; however, the implication of epigenetic mechanisms was suggested. In this study, we investigated the striatum for DNA methylation alterations under chronic levodopa treatment with or without co-treatment with Riluzole. Our data show that the lesioned and contralateral striata have nearly identical DNA methylation profiles. Chronic levodopa and levodopa + Riluzole treatments led to DNA methylation loss, particularly outside of promoters, in gene bodies and CpG poor regions. We observed that several genes involved in the Levodopa-Induced Dyskinesia underwent methylation changes. Furthermore, the Riluzole co-treatment, which improved the phenotype, pinpointed specific methylation targets, with a more than 20% methylation difference relative to levodopa treatment alone. These findings indicate potential new druggable targets for Levodopa-Induced Dyskinesia.
Keywords: DNA methylation; Reduced Representation Bisulfite Sequencing (RRBS); Riluzole; Tourette syndrome; abnormal involuntary movements; dyskinesia; epigenetics; levodopa.
Conflict of interest statement
This study has been performed in part in Boehringer Ingelheim Pharma GmbH & Co. KG laboratories. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
- FP7-People-2012-ITN TS-Eurotrain 316978/H2020 Marie Skłodowska-Curie Actions
- EuroCellNet COST (CA15214)/Horizon 2020
- Merit-prize/Semmelweis Egyetem
- Bolyai Janos BO/00987/16/5 and BO/00730/19/8/Magyar Tudományos Akadémia
- ÚNKP-18-4 and UNKP-2020 New National Excellence Program/Emberi Eroforrások Minisztériuma
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

