Systemic Administration of Insulin Receptor Antagonist Results in Endothelial and Perivascular Adipose Tissue Dysfunction in Mice
- PMID: 34207844
- PMCID: PMC8230211
- DOI: 10.3390/cells10061448
Systemic Administration of Insulin Receptor Antagonist Results in Endothelial and Perivascular Adipose Tissue Dysfunction in Mice
Abstract
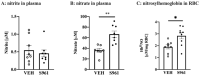
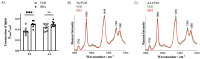
Hyperglycemia linked to diabetes results in endothelial dysfunction. In the present work, we comprehensively characterized effects of short-term hyperglycemia induced by administration of an insulin receptor antagonist, the S961 peptide, on endothelium and perivascular adipose tissue (PVAT) in mice. Endothelial function of the thoracic and abdominal aorta in 12-week-old male C57Bl/6Jrj mice treated for two weeks with S961 infusion via osmotic pumps was assessed in vivo using magnetic resonance imaging and ex vivo by detection of nitric oxide (NO) production using electron paramagnetic resonance spectroscopy. Additional methods were used to analyze PVAT, aortic segments and endothelial-specific plasma biomarkers. Systemic disruption of insulin signaling resulted in severe impairment of NO-dependent endothelial function and a loss of vasoprotective function of PVAT affecting the thoracic as well as abdominal parts of the aorta, however a fall in adiponectin expression and decreased uncoupling protein 1-positive area were more pronounced in the thoracic aorta. Results suggest that dysfunctional PVAT contributes to vascular pathology induced by altered insulin signaling in diabetes, in the absence of fat overload and obesity.
Keywords: endothelial function; insulin receptor antagonist; magnetic resonance imaging; perivascular adipose tissue.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures




Similar articles
-
Irisin Regulates Heme Oxygenase-1/Adiponectin Axis in Perivascular Adipose Tissue and Improves Endothelial Dysfunction in Diet-Induced Obese Mice.Cell Physiol Biochem. 2017;42(2):603-614. doi: 10.1159/000477864. Epub 2017 Jun 9. Cell Physiol Biochem. 2017. PMID: 28595178
-
In Vivo Magnetic Resonance Imaging-Based Detection of Heterogeneous Endothelial Response in Thoracic and Abdominal Aorta to Short-Term High-Fat Diet Ascribed to Differences in Perivascular Adipose Tissue in Mice.J Am Heart Assoc. 2020 Nov 3;9(21):e016929. doi: 10.1161/JAHA.120.016929. Epub 2020 Oct 19. J Am Heart Assoc. 2020. PMID: 33073641 Free PMC article.
-
Lack of AMP-activated protein kinase-α1 reduces nitric oxide synthesis in thoracic aorta perivascular adipose tissue.Vascul Pharmacol. 2024 Dec;157:107437. doi: 10.1016/j.vph.2024.107437. Epub 2024 Oct 20. Vascul Pharmacol. 2024. PMID: 39433170
-
Endothelial Dysfunction in Obesity.Adv Exp Med Biol. 2017;960:345-379. doi: 10.1007/978-3-319-48382-5_15. Adv Exp Med Biol. 2017. PMID: 28585207 Review.
-
Endothelial dysfunction and diabetes: roles of hyperglycemia, impaired insulin signaling and obesity.Cell Tissue Res. 2009 Jan;335(1):165-89. doi: 10.1007/s00441-008-0685-6. Epub 2008 Oct 22. Cell Tissue Res. 2009. PMID: 18941783 Review.
Cited by
-
Rapid shear stress-dependent ENaC membrane insertion is mediated by the endothelial glycocalyx and the mineralocorticoid receptor.Cell Mol Life Sci. 2022 Apr 10;79(5):235. doi: 10.1007/s00018-022-04260-y. Cell Mol Life Sci. 2022. PMID: 35397686 Free PMC article.
-
Phylloquinone improves endothelial function, inhibits cellular senescence, and vascular inflammation.Geroscience. 2024 Oct;46(5):4909-4935. doi: 10.1007/s11357-024-01225-w. Epub 2024 Jul 9. Geroscience. 2024. PMID: 38980631 Free PMC article.
-
Placental Fatty Acid Metabolism and Transport in a Rat Model of Gestational Diabetes Mellitus.J Womens Health Dev. 2023;6(2):56-67. doi: 10.26502/fjwhd.2644-288400108. Epub 2023 May 4. J Womens Health Dev. 2023. PMID: 37288271 Free PMC article.
-
Skin Microhemodynamics and Mechanisms of Its Regulation in Type 2 Diabetes Mellitus.Biophysics (Oxf). 2022;67(4):647-659. doi: 10.1134/S0006350922040200. Epub 2022 Oct 19. Biophysics (Oxf). 2022. PMID: 36281313 Free PMC article.
-
Perivascular adipose tissue: a central player in the triad of diabetes, obesity, and cardiovascular health.Cardiovasc Diabetol. 2024 Dec 28;23(1):455. doi: 10.1186/s12933-024-02549-9. Cardiovasc Diabetol. 2024. PMID: 39732729 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

