Exploiting Protein N-Terminus for Site-Specific Bioconjugation
- PMID: 34207845
- PMCID: PMC8228110
- DOI: 10.3390/molecules26123521
Exploiting Protein N-Terminus for Site-Specific Bioconjugation
Abstract
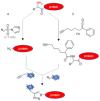
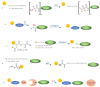
Although a plethora of chemistries have been developed to selectively decorate protein molecules, novel strategies continue to be reported with the final aim of improving selectivity and mildness of the reaction conditions, preserve protein integrity, and fulfill all the increasing requirements of the modern applications of protein conjugates. The targeting of the protein N-terminal alpha-amine group appears a convenient solution to the issue, emerging as a useful and unique reactive site universally present in each protein molecule. Herein, we provide an updated overview of the methodologies developed until today to afford the selective modification of proteins through the targeting of the N-terminal alpha-amine. Chemical and enzymatic strategies enabling the selective labeling of the protein N-terminal alpha-amine group are described.
Keywords: aldehyde protein; azide protein; chemical ligation; chemo-selective reaction; click-chemistry; molecular probe; protein labeling.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Shadish J.A., DeForest C.A. Site-selective protein modification: From functionalized proteins to functional biomaterials. Matter-Us. 2020;2:50–77. doi: 10.1016/j.matt.2019.11.011. - DOI

