Antibody-Drug Conjugates for the Treatment of Breast Cancer
- PMID: 34207890
- PMCID: PMC8229763
- DOI: 10.3390/cancers13122898
Antibody-Drug Conjugates for the Treatment of Breast Cancer
Abstract
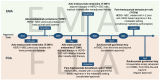
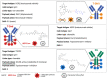
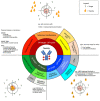
Metastatic breast cancer (BC) is currently an incurable disease. Besides endocrine therapy and targeted agents, chemotherapy is often used in the treatment of this disease. However, lack of tumor specificity and toxicity associated with dose exposure limit the manageability of cytotoxic agents. Antibody-drug conjugates (ADCs) are a relatively new class of anticancer drugs. By merging the selectivity of monoclonal antibodies with the cytotoxic properties of chemotherapy, they improve the therapeutic index of antineoplastic agents. Three core components characterize ADCs: the antibody, directed to a target antigen; the payload, typically a cytotoxic agent; a linker, connecting the antibody to the payload. The most studied target antigen is HER2 with some agents, such as trastuzumab deruxtecan, showing activity not only in HER2-positive, but also in HER2-low BC patients, possibly due to a bystander effect. This property to provide a cytotoxic impact also against off-target cancer cells may overcome the intratumoral heterogeneity of some target antigens. Other cancer-associated antigens represent a strategy for the development of ADCs against triple-negative BC, as shown by the recent approval of sacituzumab govitecan. In this review, we discuss the current landscape of ADC development for the treatment of BC, as well as the possible limitations of this treatment.
Keywords: ADCs; antibody; antibody–drug conjugates; breast cancer; target therapy.
Conflict of interest statement
C.C., F.G., E.N. and L.A. have no potential conflicts of interest to disclose. G.C. served as consultant or advisor for Roche, Lilly and Bristol-Myers Squibb, served on the speaker’s bureau for Roche, Pfizer and Lilly, received travel funding from Pfizer and Roche and received honoraria from Roche, Pfizer, Lilly, Novartis and SEAGEN, all outside the submitted work.
Figures

References
-
- Cardoso F., Paluch-Shimon S., Senkus E., Curigliano G., Aapro M.S., André F., Barrios C.H., Bergh J., Bhattacharyya G.S., Biganzoli L., et al. 5th ESO-ESMO international consensus guidelines for advanced breast cancer (ABC 5) Ann. Oncol. 2020;31:1623–1649. doi: 10.1016/j.annonc.2020.09.010. - DOI - PMC - PubMed
-
- Mathe G., Tran Ba L.O., Bernard J. Effect on mouse leukemia 1210 of a combination by diazo-reaction of amethopterin and gamma-globulins from hamsters inoculated with such leukemia by heterografts. Comptes Rendus Hebd. Seances Acad. Sci. 1958;246:1626–1628. - PubMed
-
- Elias D.J., Hirschowitz L., Kline L.E., Kroener J.F., Dillman R.O., Walker L.E., Robb J.A., Timms R.M. Phase I clinical comparative study of monoclonal antibody KS1/4 and KS1/4-methotrexate immunconjugate in patients with non-small cell lung carcinoma. Cancer Res. 1990;50:4154–4159. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

