An Examination of the Long-Term Neurodevelopmental Impact of Prenatal Zika Virus Infection in a Rat Model Using a High Resolution, Longitudinal MRI Approach
- PMID: 34207958
- PMCID: PMC8230645
- DOI: 10.3390/v13061123
An Examination of the Long-Term Neurodevelopmental Impact of Prenatal Zika Virus Infection in a Rat Model Using a High Resolution, Longitudinal MRI Approach
Abstract
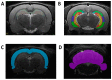
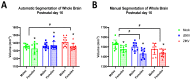
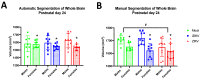
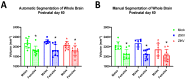
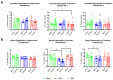
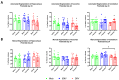
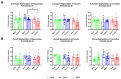
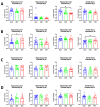
Since Zika virus (ZIKV) first emerged as a public health concern in 2015, our ability to identify and track the long-term neurological sequelae of prenatal Zika virus (ZIKV) infection in humans has been limited. Our lab has developed a rat model of maternal ZIKV infection with associated vertical transmission to the fetus that results in significant brain malformations in the neonatal offspring. Here, we use this model in conjunction with longitudinal magnetic resonance imaging (MRI) to expand our understanding of the long-term neurological consequences of prenatal ZIKV infection in order to identify characteristic neurodevelopmental changes and track them across time. We exploited both manual and automated atlas-based segmentation of MR images in order to identify long-term structural changes within the developing rat brain following inoculation. The paradigm involved scanning three cohorts of male and female rats that were prenatally inoculated with 107 PFU ZIKV, 107 UV-inactivated ZIKV (iZIKV), or diluent medium (mock), at 4 different postnatal day (P) age points: P2, P16, P24, and P60. Analysis of tracked brain structures revealed significantly altered development in both the ZIKV and iZIKV rats. Moreover, we demonstrate that prenatal ZIKV infection alters the growth of brain regions throughout the neonatal and juvenile ages. Our findings also suggest that maternal immune activation caused by inactive viral proteins may play a role in altered brain growth throughout development. For the very first time, we introduce manual and automated atlas-based segmentation of neonatal and juvenile rat brains longitudinally. Experimental results demonstrate the effectiveness of our novel approach for detecting significant changes in neurodevelopment in models of early-life infections.
Keywords: MRI; Zika virus (ZIKV); congenital infection; neurodevelopment; neuroimaging; pregnancy.
Conflict of interest statement
P.K. has financial interests in Ekam Solutions, the company that developed the Ekam Visualization and Analysis (EVA) tool used in this study. The other authors declare no conflicts of interest.
Figures


References
-
- Mann T.Z., Haddad L.B., Williams T.R., Hills S.L., Read J.S., Dee D.L., Dziuban E.J., Pérez-Padilla J., Jamieson D.J., Honein M.A., et al. Breast Milk Transmission of Flaviviruses in the Context of Zika Virus: A Systematic Review. Paediatr. Perinat. Epidemiol. 2018;32:358–368. doi: 10.1111/ppe.12478. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

