Regulation of RAD51 at the Transcriptional and Functional Levels: What Prospects for Cancer Therapy?
- PMID: 34208195
- PMCID: PMC8230762
- DOI: 10.3390/cancers13122930
Regulation of RAD51 at the Transcriptional and Functional Levels: What Prospects for Cancer Therapy?
Abstract
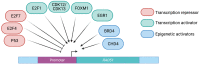
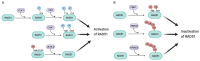
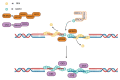
The RAD51 recombinase is a critical effector of Homologous Recombination (HR), which is an essential DNA repair mechanism for double-strand breaks. The RAD51 protein is recruited onto the DNA break by BRCA2 and forms homopolymeric filaments that invade the homologous chromatid and use it as a template for repair. RAD51 filaments are detectable by immunofluorescence as distinct foci in the cell nucleus, and their presence is a read out of HR proficiency. RAD51 is an essential gene, protecting cells from genetic instability. Its expression is low and tightly regulated in normal cells and, contrastingly, elevated in a large fraction of cancers, where its level of expression and activity have been linked with sensitivity to genotoxic treatment. In particular, BRCA-deficient tumors show reduced or obliterated RAD51 foci formation and increased sensitivity to platinum salt or PARP inhibitors. However, resistance to treatment sets in rapidly and is frequently based on a complete or partial restoration of RAD51 foci formation. Consequently, RAD51 could be a highly valuable therapeutic target. Here, we review the multiple levels of regulation that impact the transcription of the RAD51 gene, as well as the post-translational modifications that determine its expression level, recruitment on DNA damage sites and the efficient formation of homofilaments. Some of these regulation levels may be targeted and their impact on cancer cell survival discussed.
Keywords: DNA break; DNA repair; RAD51; cancer therapy; genomic instability; homologous recombination.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Póti Á., Gyergyák H., Németh E., Rusz O., Tóth S., Kovácsházi C., Chen D., Szikriszt B., Spisák S., Takeda S., et al. Correlation of homologous recombination deficiency induced mutational signatures with sensitivity to PARP inhibitors and cytotoxic agents. Genome Biol. 2019;20:240. doi: 10.1186/s13059-019-1867-0. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

