Two Approaches to the Laser-Induced Formation of Au/Ag Bimetallic Nanoparticles in Supercritical Carbon Dioxide
- PMID: 34208329
- PMCID: PMC8231236
- DOI: 10.3390/nano11061553
Two Approaches to the Laser-Induced Formation of Au/Ag Bimetallic Nanoparticles in Supercritical Carbon Dioxide
Abstract
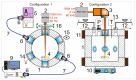
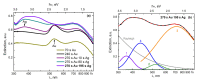
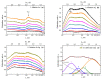
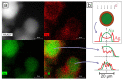
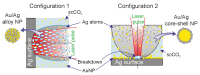
Two approaches are proposed for the synthesis of bimetallic Au/Ag nanoparticles, using the pulsed laser ablation of a target consisting of gold and silver plates in a medium of supercritical carbon dioxide. The differences between the two approaches related to the field of "green chemistry" are in the use of different geometric configurations and different laser sources when carrying out the experiments. In the first configuration, the Ag and Au targets are placed side-by-side vertically on the side wall of a high-pressure reactor and the ablation of the target plates occurs alternately with a stationary "wide" horizontal beam with a laser pulse repetition rate of 50 Hz. In the second configuration, the targets are placed horizontally at the bottom of a reactor and the ablation of their parts is carried out by scanning from above with a vertical "narrow" laser beam with a pulse repetition rate of 60 kHz. The possibility of obtaining Ag/Au alloy nanoparticles is demonstrated using the first configuration, while the possibility of obtaining "core-shell" bimetallic Au/Ag nanoparticles with a gold core and a silver shell is demonstrated using the second configuration. A simple model is proposed to explain the obtained results.
Keywords: laser ablation; plasmonic nanoparticles; supercritical carbon dioxide; supercritical fluid.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Eng N.B. Synergetic Antibacterial Effects of Silver Nanoparticles @ Aloe Vera Prepared via a Green Method. Nano Biomed. Eng. 2010;2:267–274. doi: 10.5101/nbe.v2i4.p252-257.1. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

