Fabrication and Characterization of Fast-Dissolving Films Containing Escitalopram/Quetiapine for the Treatment of Major Depressive Disorder
- PMID: 34208460
- PMCID: PMC8234593
- DOI: 10.3390/pharmaceutics13060891
Fabrication and Characterization of Fast-Dissolving Films Containing Escitalopram/Quetiapine for the Treatment of Major Depressive Disorder
Abstract
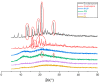
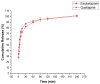
Major depressive disorder (MMD) is a leading cause of disability worldwide. Approximately one-third of patients with MDD fail to achieve response or remission leading to treatment-resistant depression (TRD). One of the psychopharmacological strategies to overcome TRD is using a combination of an antipsychotic as an augmenting agent with selective serotonin reuptake inhibitors (SSRIs). Among which, an atypical antipsychotic, quetiapine (QUE), and an SSRI, escitalopram (ESC), were formulated as a fixed-dose combination as a fast-dissolving film by coaxial electrospinning. The resultant fiber's morphology was studied. SEM images showed that the drug-loaded fibers were smooth, un-beaded, and non-porous with a fiber diameter of 0.9 ± 0.1 µm, while the TEM images illustrated the distinctive layers of the core and shell, confirming the successful preparation of these fibers. Differential scanning calorimetry (DSC) and X-ray diffraction (XRD) studies confirmed that both drugs were amorphously distributed within the drug-loaded fibers. The drug-loaded fibers exhibited a disintegration time of 2 s, which accelerated the release of both drugs (50% after 5 min) making it an attractive formulation for oral mucosal delivery. The ex vivo permeability study demonstrated that QUE was permeated through the buccal membrane, but not ESC that might be hindered by the buccal epithelium and the intercellular lipids. Overall, the developed coaxial fibers could be a potential buccal dosage form that could be attributed to higher acceptability and adherence among vulnerable patients, particularly mentally ill patients.
Keywords: coaxial fibers; electrospinning; escitalopram; fast-dissolving films; major depressive disorder; quetiapine; treatment-resistant depression.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







References
-
- World Health Organization Depression. [(accessed on 23 February 2021)]; Available online: https://www.who.int/news-room/fact-sheets/detail/depression.
-
- Gelenberg A.J., Freeman M.P., Markowitz J.C., Rosenbaum J.F., Thase M.E., Trivedi M.H., van Rhoads R.S., Reus V.I., DePaulo J.R., Jr., Fawcett J.A., et al. Practice Guideline for the Treatment of Patients with Major Depressive Disorder, Third Edition. Am. J. Psychiatry. 2010;167:1. - PubMed
-
- Brakemeier E.-L., Radtke M., Engel V., Zimmermann J., Tuschen-Caffier B., Hautzinger M., Schramm E., Berger M., Normann C. Overcoming Treatment Resistance in Chronic Depression: A Pilot Study on Outcome and Feasibility of the Cognitive Behavioral Analysis System of Psychotherapy as an Inpatient Treatment Program. Psychother. Psychosom. 2014;84:51–56. doi: 10.1159/000369586. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

