Sensitivity and Specificity of Human Papillomavirus (HPV) 16 Early Antigen Serology for HPV-Driven Oropharyngeal Cancer: A Systematic Literature Review and Meta-Analysis
- PMID: 34208476
- PMCID: PMC8234521
- DOI: 10.3390/cancers13123010
Sensitivity and Specificity of Human Papillomavirus (HPV) 16 Early Antigen Serology for HPV-Driven Oropharyngeal Cancer: A Systematic Literature Review and Meta-Analysis
Abstract
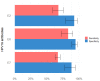
Antibodies against HPV16 early proteins have been shown to be promising biomarkers for the identification of HPV-driven oropharyngeal cancer (HPV-OPC) among OPC cases in multiple studies. A systematic literature search was performed to identify original research articles comparing HPV early antigen serology with established reference methods to determine molecular HPV tumor status. Random-effects models were used to calculate summary estimates for sensitivity and specificity of HPV16 E2, E6 and E7 serology for HPV-OPC. Subgroup analyses were performed to explore heterogeneity across studies and describe variables associated with test performance. We identified n = 23 studies meeting all eligibility criteria and included these in the meta-analysis. E6 serology showed the best performance with pooled sensitivity and specificity estimates of 83.1% (95% confidence interval (CI) 72.5-90.2%) and 94.6% (95% CI 89.0-97.4%), respectively, while E2 and E7 serological assays were highly specific (E2: 92.5% (95% CI 79.1-97.6%); E7: 88.5% (95% CI 77.9-94.4%)) but moderately sensitive (E2: 67.8% (95% CI 58.9-75.6%); E7: 67.0% (95% CI 63.2-70.6%)). Subgroup analyses revealed increased pooled sensitivity for bacterially (89.9% (95% CI 84.5-93.6%)) vs. in vitro expressed E6 antigen (55.3% (95% CI 41.0-68.7%)), while both showed high specificity (95.2% (95% CI 93.0-96.7%) and 91.1% (95% CI 46.6-99.2%), respectively). Pooled specificity estimates for HPV16 E2, E6 and E7 serology were significantly lower in studies utilizing HPV DNA PCR as the only molecular reference method compared to those using a combination of any two reference methods (HPV DNA, RNA, in situ hybridization (ISH), p16 immunohistochemistry (IHC)), or histopathological reference methods (ISH or p16 IHC) as stand-alone marker. In conclusion, HPV16 E6 seropositivity is a highly sensitive and specific biomarker for HPV-OPC. However, its performance differs between serological assays and depends on molecular reference methods.
Keywords: HPV; antibodies; meta-analysis; oropharyngeal cancer; sensitivity; serology; specificity; systematic review.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Anantharaman D., Abedi-Ardekani B., Beachler D.C., Gheit T., Olshan A.F., Wisniewski K., Wunsch-Filho V., Toporcov T.N., Tajara E.H., Levi J.E., et al. Geographic Heterogeneity in the Prevalence of Human Papillomavirus in Head and Neck Cancer. Int. J. Cancer. 2017;140:1968–1975. doi: 10.1002/ijc.30608. - DOI - PMC - PubMed
-
- Chaturvedi A.K., Engels E.A., Pfeiffer R.M., Hernandez B.Y., Xiao W., Kim E., Jiang B., Goodman M.T., Sibug-Saber M., Cozen W., et al. Human Papillomavirus and Rising Oropharyngeal Cancer Incidence in the United States. J. Clin. Oncol. 2011;29:4294–4301. doi: 10.1200/JCO.2011.36.4596. - DOI - PMC - PubMed
-
- Castellsagué X., Alemany L., Quer M., Halec G., Quirós B., Tous S., Clavero O., Alòs L., Biegner T., Szafarowski T., et al. HPV Involvement in Head and Neck Cancers: Comprehensive Assessment of Biomarkers in 3680 Patients. J. Natl. Cancer Inst. 2016;108:djv403. doi: 10.1093/jnci/djv403. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

