Solvothermal Preparation of a Lanthanide Metal-Organic Framework for Highly Sensitive Discrimination of Nitrofurantoin and l-Tyrosine
- PMID: 34208577
- PMCID: PMC8233945
- DOI: 10.3390/molecules26123673
Solvothermal Preparation of a Lanthanide Metal-Organic Framework for Highly Sensitive Discrimination of Nitrofurantoin and l-Tyrosine
Abstract
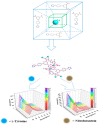
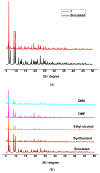
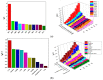
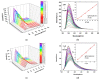
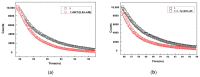
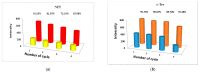
Metal-organic frameworks (MOFs) have been rapidly developed for their broad applications in many different chemistry and materials fields. In this work, a multi-dentate building block 5-(4-(tetrazol-5-yl)phenyl)-isophthalic acid (H3L) containing tetrazole and carbolxylate moieties was employed for the synthesis of a two-dimensional (2D) lanthanide MOF [La(HL)(DMF)2(NO3)] (DMF = N,N-dimethylformamide) (1) under solvothermal condition. The fluorescent sensing application of 1 was investigated. 1 exhibits high sensitivity recognition for antibiotic nitrofurantoin (Ksv: 3.0 × 103 M-1 and detection limit: 17.0 μM) and amino acid l-tyrosine (Ksv: 1.4 × 104 M-1 and detection limit: 3.6 μM). This work provides a feasible detection platform of 2D MOFs for highly sensitive discrimination of antibiotics and amino acids.
Keywords: amino acid; antibiotics; fluorescence; fluorescent probe; metal-organic frameworks.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Calahorro A.J., Briones D., Seco J.M., Salinas-Castillo A., Rodriguez-Dieguez A., Mendicute-Fierro C. Bidimensional cadmium metal-organic frameworks based on 1,3-bis(4-pyridyl)propane displaying long lifetime photoluminescence emission. Polyhedron. 2015;91:47–51. doi: 10.1016/j.poly.2015.02.025. - DOI
-
- Al-Janabi N., Deng H.R., Borges J., Liu X.F., Garforth A., Siperstein F.R., Fan X.L. A facile post-synthetic modification method to improve hydrothermal stability and CO2 selectivity of CuBTC metal−organic framework. Ind. Eng. Chem. 2016;55:7941–7949. doi: 10.1021/acs.iecr.5b04217. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

