Thiophene-Based Trimers and Their Bioapplications: An Overview
- PMID: 34208624
- PMCID: PMC8234281
- DOI: 10.3390/polym13121977
Thiophene-Based Trimers and Their Bioapplications: An Overview
Abstract
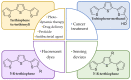
Certainly, the success of polythiophenes is due in the first place to their outstanding electronic properties and superior processability. Nevertheless, there are additional reasons that contribute to arouse the scientific interest around these materials. Among these, the large variety of chemical modifications that is possible to perform on the thiophene ring is a precious aspect. In particular, a turning point was marked by the diffusion of synthetic strategies for the preparation of terthiophenes: the vast richness of approaches today available for the easy customization of these structures allows the finetuning of their chemical, physical, and optical properties. Therefore, terthiophene derivatives have become an extremely versatile class of compounds both for direct application or for the preparation of electronic functional polymers. Moreover, their biocompatibility and ease of functionalization make them appealing for biology and medical research, as it testifies to the blossoming of studies in these fields in which they are involved. It is thus with the willingness to guide the reader through all the possibilities offered by these structures that this review elucidates the synthetic methods and describes the full chemical variety of terthiophenes and their derivatives. In the final part, an in-depth presentation of their numerous bioapplications intends to provide a complete picture of the state of the art.
Keywords: biosensing; conjugated polymers; photosensitizers; polythiophenes; terthiophenes; thiophene trimers.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






























References
-
- MacDiarmid A.G., Epstein A.J. Conducting Polymers: Past, Present and Future…. MRS Proc. 1993;328:133. doi: 10.1557/PROC-328-133. - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

