Serological Response in Lung Transplant Recipients after Two Doses of SARS-CoV-2 mRNA Vaccines
- PMID: 34208884
- PMCID: PMC8310345
- DOI: 10.3390/vaccines9070708
Serological Response in Lung Transplant Recipients after Two Doses of SARS-CoV-2 mRNA Vaccines
Abstract
Background: Lung-transplant (LT) recipients are at high risk for COVID-19 due to immunosuppression and respiratory tropism of SARS-CoV-2. The information on the effect of COVID-19 mRNA vaccines to elicit immunogenic responses after a two-dose (2D) regimen in LT recipients is sparse. Thus, we assessed the effect of Pfizer-BioNTech and Moderna mRNA vaccines' 2D regimen on anti-spike responses in immunocompromised LT recipients.
Methods: We utilized serum samples from LT recipients vaccinated for SARS-CoV-2 with 2D of either the Pfizer-BioNTech or Moderna vaccines and 2D-vaccinated naïve (non-transplanted and non-exposed to COVID-19) group. Antibody responses were assessed using the FDA-approved SARS-CoV-2 anti-nucleocapsid protein IgG assay (IgGNC), the SARS-CoV-2 anti-spike protein IgM assay (IgMSP), and the SARS-CoV-2 anti-spike protein IgG II assay (IgGSP). CD4+ T-cell activity was assessed as a marker of immune competence using the ImmuKnow® assay.
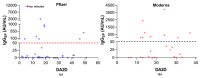
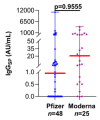
Results: About 25% (18/73) of SARS-CoV-2 uninfected-LT patients generated a positive spike-IgG response following 2D of vaccines, with 36% (9/25) in the Moderna cohort and only 19% (9/48) in the Pfizer cohort. 2D in LT patients elicited a significantly lesser median IgGSP response (1.7 AU/mL, 95% CI: 0.6-7.5 AU/mL) compared to non-transplanted, uninfected naïve subjects (14,209 AU/mL, 95% CI: 11,261-18,836 AU/mL; p < 0.0001). In LT patients, the Moderna-evoked seropositivity trend was higher than Pfizer.
Conclusion: 2D COVID-19 vaccination elicits a dampened serological response in LT patients. Whether assessing other arms of host immunity combined with a higher vaccine dose can better capture and elicit improved immunogenicity in this immunocompromised population warrants investigation.
Keywords: COVID-19 mRNA vaccines; IgG; IgM; SARS-CoV-2; Spike; lung transplantation; nucleocapsid; serological response; two doses.
Conflict of interest statement
Abbott Diagnostics, while providing a part of the antibody testing reagents, did not have any role in the study’s design, collection, analyses, or interpretation of the data; drafting the manuscript; or in the decision to publish the results. The authors declare no other conflicts of interest.
Figures

References
-
- SARS-CoV-2 Vaccination in Heart and Lung Transplantation: Recommendations from the ISHLT COVID-19 Task Force. [(accessed on 7 June 2021)]; Available online: https://ishlt.org/ishlt/media/Documents/COVID19_Vaccine-Recommendations_....
-
- Narasimhan M., Mahimainathan L., Araj E., Clark A.E., Markantonis J., Green A., Xu J., SoRelle J.A., Alexis C., Fankhauser K., et al. Clinical evaluation of the Abbott Alinity SARS-CoV-2 spike-specific quantitative IgG and IgM assays in infected, recovered, and vaccinated groups. J. Clin. Microbiol. 2021;59:e0038821. doi: 10.1128/JCM.00388-21. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

