Analysis of Dip2B Expression in Adult Mouse Tissues Using the LacZ Reporter Gene
- PMID: 34208944
- PMCID: PMC8929063
- DOI: 10.3390/cimb43020040
Analysis of Dip2B Expression in Adult Mouse Tissues Using the LacZ Reporter Gene
Abstract
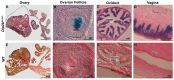
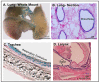
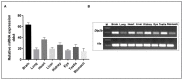
Disconnected (disco)-interacting protein 2 homolog B (Dip2B) is a member of the Dip2 superfamily and plays an essential role in axonal outgrowth during embryogenesis. In adults, Dip2B is highly expressed in different brain regions, as shown by in situ analysis, and may have a role in axon guidance. However, the expression and biological role of Dip2B in other somatic tissues remain unknown. To better visualize Dip2B expression and to provide insight into the roles of Dip2B during postnatal development, we used a Dip2btm1a(wtsi)komp knock-in mouse model, in which a LacZ-Neo fusion protein is expressed under Dip2b promoter and allowed Dip2B expression to be analyzed by X-gal staining. qPCR analyses showed that Dip2b mRNA was expressed in a variety of somatic tissues, including lung and kidney, in addition to brain. LacZ staining indicated that Dip2B is broadly expressed in neuronal, reproductive, and vascular tissues as well as in the kidneys, heart, liver, and lungs. Moreover, neurons and epithelial cells showed rich staining. The broad and intense patterns of Dip2B expression in adult mice provide evidence of the distribution of Dip2B in multiple locations and, thereby, its implication in numerous physiological roles.
Keywords: Dip2b; LacZ; nervous system; reproductive system; respiratory system; vascular system.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- Mukhopadhyay M., Pelka P., DeSousa D., Kablar B., Schindler A., Rudnicki M.A., Campos A.R. Cloning, Genomic Organization and Expression Pattern of a Novel Drosophila Gene, the Disco-Interacting Protein 2 (Dip2), and Its Murine Homolog. Gene. 2002;293:59–65. doi: 10.1016/S0378-1119(02)00694-7. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

