Nanocarriers for Biomedicine: From Lipid Formulations to Inorganic and Hybrid Nanoparticles
- PMID: 34209023
- PMCID: PMC8269010
- DOI: 10.3390/ijms22137055
Nanocarriers for Biomedicine: From Lipid Formulations to Inorganic and Hybrid Nanoparticles
Abstract
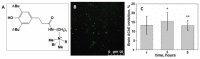
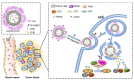
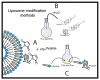
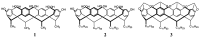
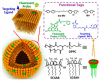
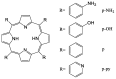
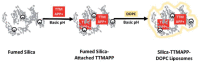
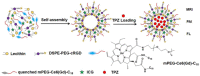
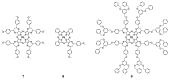
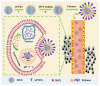
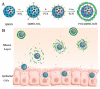
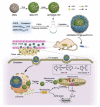
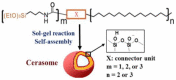
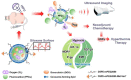
Encapsulation of cargoes in nanocontainers is widely used in different fields to solve the problems of their solubility, homogeneity, stability, protection from unwanted chemical and biological destructive effects, and functional activity improvement. This approach is of special importance in biomedicine, since this makes it possible to reduce the limitations of drug delivery related to the toxicity and side effects of therapeutics, their low bioavailability and biocompatibility. This review highlights current progress in the use of lipid systems to deliver active substances to the human body. Various lipid compositions modified with amphiphilic open-chain and macrocyclic compounds, peptide molecules and alternative target ligands are discussed. Liposome modification also evolves by creating new hybrid structures consisting of organic and inorganic parts. Such nanohybrid platforms include cerasomes, which are considered as alternative nanocarriers allowing to reduce inherent limitations of lipid nanoparticles. Compositions based on mesoporous silica are beginning to acquire no less relevance due to their unique features, such as advanced porous properties, well-proven drug delivery efficiency and their versatility for creating highly efficient nanomaterials. The types of silica nanoparticles, their efficacy in biomedical applications and hybrid inorganic-polymer platforms are the subject of discussion in this review, with current challenges emphasized.
Keywords: cerasome; drug delivery; hybrid nanocarriers; liposome; macrocycle; mesoporous silica; non-covalent modification; peptide; surfactant.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


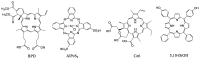

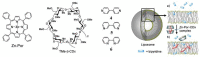

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

