Oral Ferric Maltol Does Not Adversely Affect the Intestinal Microbiome of Patients or Mice, But Ferrous Sulphate Does
- PMID: 34209042
- PMCID: PMC8308237
- DOI: 10.3390/nu13072269
Oral Ferric Maltol Does Not Adversely Affect the Intestinal Microbiome of Patients or Mice, But Ferrous Sulphate Does
Abstract
Background and aims: Altering dietary ferrous sulphate (FS) consumption exacerbates a murine model of colitis and alters the intestinal microbiome. We investigated the impact of oral ferric maltol (FM) and FS on mice with dextran sodium sulphate (DSS) induced colitis, and the microbiome of patients with iron deficiency.
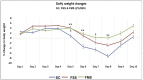
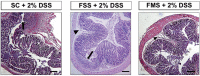
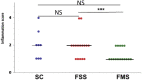
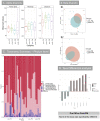
Methods: Mice had acute colitis induced, with 2% DSS for 5 days, followed by water. During this period, groups of mice were fed standard chow (200 ppm iron, SC, n = 8), or SC with 200ppm FS supplementation (n = 16, FSS), or SC with 200 ppm FM supplementation (n = 16, FMS). Clinical, pathological and microbiome assessments were compared at days 1 and 10. Fecal bacterial gDNA was extracted and the microbiome assessed by sequencing. Statistical inferences were made using MacQIIME. Principal Coordinates Analysis were used to visualize beta-diversity cluster analysis. Ten patients with IDA were treated with FS, and six with inactive inflammatory bowel disease received FM, supplements for four weeks: pre- and mid-treatment fecal samples were collected: the microbiome was assessed (see above).
Results: In mice, after DSS treatment, there was a decrease in many genera in the SC and FSS groups: Lactobacillales increased in mice that received FMS. In humans, FS treatment led to an increase in five genera, but FM was not associated with any measurable change. The severity of DSS-induced colitis was greater with FSS than FMS.
Conclusions: This study demonstrates differential and unique influences of ferric maltol and ferrous sulphate supplements on intestinal microbiota. These differences might contribute to the different side effects associated with these preparations.
Keywords: dysbiosis; iron; microbiome.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Stein J., Bager P., Befrits R., Gasche C., Gudehus M., Lerebours E., Magro F., Mearin F., Mitchell D., Oldenburg B., et al. Anaemia management in patients with inflammatory bowel disease: Routine practice across nine European countries. Eur. J. Gastroenterol. Hepatol. 2013;25:1456–1463. doi: 10.1097/MEG.0b013e328365ca7f. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

