The Neuroinflammatory Role of Pericytes in Epilepsy
- PMID: 34209145
- PMCID: PMC8301485
- DOI: 10.3390/biomedicines9070759
The Neuroinflammatory Role of Pericytes in Epilepsy
Abstract
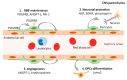
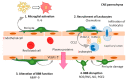
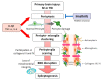
Pericytes are a component of the blood-brain barrier (BBB) neurovascular unit, in which they play a crucial role in BBB integrity and are also implicated in neuroinflammation. The association between pericytes, BBB dysfunction, and the pathophysiology of epilepsy has been investigated, and links between epilepsy and pericytes have been identified. Here, we review current knowledge about the role of pericytes in epilepsy. Clinical evidence has shown an accumulation of pericytes with altered morphology in the cerebral vascular territories of patients with intractable epilepsy. In vitro, proinflammatory cytokines, including IL-1β, TNFα, and IL-6, cause morphological changes in human-derived pericytes, where IL-6 leads to cell damage. Experimental studies using epileptic animal models have shown that cerebrovascular pericytes undergo redistribution and remodeling, potentially contributing to BBB permeability. These series of pericyte-related modifications are promoted by proinflammatory cytokines, of which the most pronounced alterations are caused by IL-1β, a cytokine involved in the pathogenesis of epilepsy. Furthermore, the pericyte-glial scarring process in leaky capillaries was detected in the hippocampus during seizure progression. In addition, pericytes respond more sensitively to proinflammatory cytokines than microglia and can also activate microglia. Thus, pericytes may function as sensors of the inflammatory response. Finally, both in vitro and in vivo studies have highlighted the potential of pericytes as a therapeutic target for seizure disorders.
Keywords: blood-brain barrier; cytokine; mural cells; neuroinflammation; pericytes.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Seizure progression and inflammatory mediators promote pericytosis and pericyte-microglia clustering at the cerebrovasculature.Neurobiol Dis. 2018 May;113:70-81. doi: 10.1016/j.nbd.2018.02.002. Epub 2018 Feb 9. Neurobiol Dis. 2018. PMID: 29432809
-
Pericytes and Microglia: Neurovascular and Immune Regulatory Cells in Seizure Disorders.In: Noebels JL, Avoli M, Rogawski MA, Vezzani A, Delgado-Escueta AV, editors. Jasper's Basic Mechanisms of the Epilepsies. 5th edition. New York: Oxford University Press; 2024. Chapter 29. In: Noebels JL, Avoli M, Rogawski MA, Vezzani A, Delgado-Escueta AV, editors. Jasper's Basic Mechanisms of the Epilepsies. 5th edition. New York: Oxford University Press; 2024. Chapter 29. PMID: 39637115 Free Books & Documents. Review.
-
Unique and shared inflammatory profiles of human brain endothelia and pericytes.J Neuroinflammation. 2018 May 11;15(1):138. doi: 10.1186/s12974-018-1167-8. J Neuroinflammation. 2018. PMID: 29751771 Free PMC article.
-
Seizure-induced microvascular injury is associated with impaired neurovascular coupling and blood-brain barrier dysfunction.Epilepsia. 2019 Feb;60(2):322-336. doi: 10.1111/epi.14631. Epub 2019 Jan 4. Epilepsia. 2019. PMID: 30609012
-
Brain Microvascular Pericytes in Vascular Cognitive Impairment and Dementia.Front Aging Neurosci. 2020 Apr 14;12:80. doi: 10.3389/fnagi.2020.00080. eCollection 2020. Front Aging Neurosci. 2020. PMID: 32317958 Free PMC article. Review.
Cited by
-
Pericyte derivation and transplantation for blood-CNS barrier reconstitution in CNS disorders.IBRO Neurosci Rep. 2024 Jan 3;16:147-154. doi: 10.1016/j.ibneur.2023.12.007. eCollection 2024 Jun. IBRO Neurosci Rep. 2024. PMID: 39007089 Free PMC article.
-
CfDNA Measurement as a Diagnostic Tool for the Detection of Brain Somatic Mutations in Refractory Epilepsy.Int J Mol Sci. 2022 Apr 28;23(9):4879. doi: 10.3390/ijms23094879. Int J Mol Sci. 2022. PMID: 35563270 Free PMC article. Review.
-
Mechanistic insights into the sleep-glymphopathy-cerebral small vessel disease loop: implications for epilepsy pathophysiology and therapy.Front Neurosci. 2025 Mar 27;19:1546482. doi: 10.3389/fnins.2025.1546482. eCollection 2025. Front Neurosci. 2025. PMID: 40212714 Free PMC article. Review.
-
Analyzing pericytes under mild traumatic brain injury using 3D cultures and dielectric elastomer actuators.Front Neurosci. 2022 Nov 10;16:994251. doi: 10.3389/fnins.2022.994251. eCollection 2022. Front Neurosci. 2022. PMID: 36440264 Free PMC article.
-
The neurovasculature as a target in temporal lobe epilepsy.Brain Pathol. 2023 Mar;33(2):e13147. doi: 10.1111/bpa.13147. Epub 2023 Jan 4. Brain Pathol. 2023. PMID: 36599709 Free PMC article. Review.
References
-
- Milesi S., Boussadia B., Plaud C., Catteau M., Rousset M.C., De Bock F., Schaeffer M., Lerner-Natoli M., Rigau V., Marchi N. Redistribution of PDGFRβ cells and NG2DsRed pericytes at the cerebrovasculature after status epilepticus. Neurobiol. Dis. 2014;71:151–158. doi: 10.1016/j.nbd.2014.07.010. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

