The Interplay of Apoes with Syndecans in Influencing Key Cellular Events of Amyloid Pathology
- PMID: 34209175
- PMCID: PMC8268055
- DOI: 10.3390/ijms22137070
The Interplay of Apoes with Syndecans in Influencing Key Cellular Events of Amyloid Pathology
Abstract
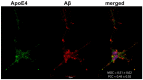
Apolipoprotein E (ApoE) isoforms exert intricate effects on cellular physiology beyond lipid transport and metabolism. ApoEs influence the onset of Alzheimer's disease (AD) in an isoform-dependent manner: ApoE4 increases AD risk, while ApoE2 decreases it. Previously we demonstrated that syndecans, a transmembrane proteoglycan family with increased expression in AD, trigger the aggregation and modulate the cellular uptake of amyloid beta (Aβ). Utilizing our previously established syndecan-overexpressing cellular assays, we now explore how the interplay of ApoEs with syndecans contributes to key events, namely uptake and aggregation, in Aβ pathology. The interaction of ApoEs with syndecans indicates isoform-specific characteristics arising beyond the frequently studied ApoE-heparan sulfate interactions. Syndecans, and among them the neuronal syndecan-3, increased the cellular uptake of ApoEs, especially ApoE2 and ApoE3, while ApoEs exerted opposing effects on syndecan-3-mediated Aβ uptake and aggregation. ApoE2 increased the cellular internalization of monomeric Aβ, hence preventing its extracellular aggregation, while ApoE4 decreased it, thus helping the buildup of extracellular plaques. The contrary effects of ApoE2 and ApoE4 remained once Aβ aggregated: while ApoE2 reduced the uptake of Aβ aggregates, ApoE4 facilitated it. Fibrillation studies also revealed ApoE4's tendency to form fibrillar aggregates. Our results uncover yet unknown details of ApoE cellular biology and deepen our molecular understanding of the ApoE-dependent mechanism of Aβ pathology.
Keywords: ApoE; amyloid beta; endocytosis; neurodegeneration; protein aggregation; syndecans.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

