Ancillary Diagnostic Investigations in Malignant Pleural Mesothelioma
- PMID: 34209209
- PMCID: PMC8268996
- DOI: 10.3390/cancers13133291
Ancillary Diagnostic Investigations in Malignant Pleural Mesothelioma
Abstract
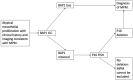
For a number of patients presenting with an undiagnosed pleural effusion, frailty, medical co-morbidity or personal choice may preclude the use of pleural biopsy, the gold standard investigation for diagnosis of malignant pleural mesothelioma (MPM). In this review article, we outline the most recent evidence on ancillary diagnostic tests which may be used to support a diagnosis of MPM where histological samples cannot be obtained or where results are non-diagnostic. Immunocytochemical markers, molecular techniques, diagnostic biomarkers and imaging techniques are discussed. No adjunctive test has a sensitivity and specificity profile to support use in isolation; however, correlation of pleural fluid cytology with relevant radiology and supplementary biomarkers can enable an MDT-consensus clinico-radiological-cytological diagnosis to be made where further invasive tests are not possible or not appropriate. Diagnostic challenges surrounding non-epithelioid MPM are recognised, and there is a critical need for reliable and non-invasive investigative tools in this population.
Keywords: biomarkers; malignant pleural mesothelioma; pleural effusion.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Woolhouse I., Bishop L., Darlison L., De Fonseka D., Edey A., Edwards J., Faivre-Finn C., Fennell D.A., Holmes S., Kerr K.M., et al. British Thoracic Society Guideline for the investigation and management of malignant pleural mesothelioma. Thorax. 2018;73:i1–i30. doi: 10.1136/thoraxjnl-2017-211321. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

