Emerging Evidence for Pleiotropism of Eosinophils
- PMID: 34209213
- PMCID: PMC8269185
- DOI: 10.3390/ijms22137075
Emerging Evidence for Pleiotropism of Eosinophils
Abstract
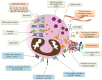
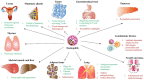
Eosinophils are complex granulocytes with the capacity to react upon diverse stimuli due to their numerous and variable surface receptors, which allows them to respond in very different manners. Traditionally believed to be only part of parasitic and allergic/asthmatic immune responses, as scientific studies arise, the paradigm about these cells is continuously changing, adding layers of complexity to their roles in homeostasis and disease. Developing principally in the bone marrow by the action of IL-5 and granulocyte macrophage colony-stimulating factor GM-CSF, eosinophils migrate from the blood to very different organs, performing multiple functions in tissue homeostasis as in the gastrointestinal tract, thymus, uterus, mammary glands, liver, and skeletal muscle. In organs such as the lungs and gastrointestinal tract, eosinophils are able to act as immune regulatory cells and also to perform direct actions against parasites, and bacteria, where novel mechanisms of immune defense as extracellular DNA traps are key factors. Besides, eosinophils, are of importance in an effective response against viral pathogens by their nuclease enzymatic activity and have been lately described as involved in severe acute respiratory syndrome coronavirus SARS-CoV-2 immunity. The pleiotropic role of eosinophils is sustained because eosinophils can be also detrimental to human physiology, for example, in diseases like allergies, asthma, and eosinophilic esophagitis, where exosomes can be significant pathophysiologic units. These eosinophilic pathologies, require specific treatments by eosinophils control, such as new monoclonal antibodies like mepolizumab, reslizumab, and benralizumab. In this review, we describe the roles of eosinophils as effectors and regulatory cells and their involvement in pathological disorders and treatment.
Keywords: biologic treatment; eosinophil; eosinophil extracellular traps; sub-phenotypes.
Conflict of interest statement
V.d.P. has received honoraria (advisory board, speaker) and/or institutional grant/research support from Astra-Zeneca and GSK. The rest of authors declare no conflict of interest.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

