Use of Rv0222-Rv2657c-Rv1509 Fusion Protein to Improve the Accuracy of an Antibody ELISA for Extra-Pulmonary Tuberculosis in Humans
- PMID: 34209358
- PMCID: PMC8308687
- DOI: 10.3390/pathogens10070828
Use of Rv0222-Rv2657c-Rv1509 Fusion Protein to Improve the Accuracy of an Antibody ELISA for Extra-Pulmonary Tuberculosis in Humans
Abstract
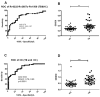
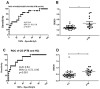
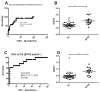
(1) Background: Tuberculosis (TB) in humans is a serious chronic epidemic disease caused by Mycobacterium tuberculosis (M. tb). The diagnosis of TB, especially extra-pulmonary TB (EPTB), is difficult. Isolation of M. tb from culture has a low sensitivity in patients with TB and an even lower sensitivity in cases of EPTB. Although Xpert MTB/RIF assays and serological tests are more sensitive than the above tests, they still lack sensitivity for EPTB diagnosis. (2) Methods: To improve the accuracy of TB diagnosis, a Rv0222-Rv2657c-Rv1509 fusion protein based iELISA was constructed, the diagnosis of TB, pulmonary TB (PTB) and EPTB was then evaluated. Sera of 40 TB patients including 14 with PTB, 14 with EPTB and 12 with no information about the form of TB, and five pneumonia patients were investigated. (3) Results: The sensitivity of the ELISA in TB, PTB and EPTB patients was 80% (95% CI: 64.4, 90.9%), 85.7% (95% CI: 57.2, 98.2%) and 92.8% (95% CI: 66.1, 99.8%), respectively, with a specificity of 70% (95% CI: 53.5, 83.4%). Both the sensitivity and specificity with this fusion protein were higher than for CFP10/ESAT6 (used as reference antigen) fusion protein (71.4%; 95% CI: 41.9, 91.6%, and 67.5%; 95% CI: 50.9, 81.4%), respectively, in cases of EPTB. All pneumonia patients' sera tested negative in both ELISAs. (4) Conclusion: use of these new fusion proteins as antigens in serological assays has the potential to improve the diagnosis of all forms of TB in humans, especially EPTB.
Keywords: diagnosis; extra-pulmonary tuberculosis; fusion protein; pulmonary tuberculosis; tuberculosis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Identification of new diagnostic biomarkers for Mycobacterium tuberculosis and the potential application in the serodiagnosis of human tuberculosis.Microb Biotechnol. 2018 Sep;11(5):893-904. doi: 10.1111/1751-7915.13291. Epub 2018 Jun 27. Microb Biotechnol. 2018. PMID: 29952084 Free PMC article.
-
Low diagnostic accuracy of Xpert MTB/RIF assay for extrapulmonary tuberculosis: A multicenter surveillance.Sci Rep. 2019 Dec 6;9(1):18515. doi: 10.1038/s41598-019-55112-y. Sci Rep. 2019. PMID: 31811239 Free PMC article.
-
A retrospective study on Xpert MTB/RIF for detection of tuberculosis in a teaching hospital in China.BMC Infect Dis. 2020 May 24;20(1):362. doi: 10.1186/s12879-020-05004-8. BMC Infect Dis. 2020. PMID: 32448123 Free PMC article.
-
Diagnostic accuracy of the new Xpert MTB/RIF Ultra for tuberculosis disease: A preliminary systematic review and meta-analysis.Int J Infect Dis. 2020 Jan;90:35-45. doi: 10.1016/j.ijid.2019.09.016. Epub 2019 Sep 20. Int J Infect Dis. 2020. PMID: 31546008
-
Diagnostic accuracy of the Xpert® MTB/RIF assay for extra-pulmonary tuberculosis: a meta-analysis.Int J Tuberc Lung Dis. 2015 Mar;19(3):278-84, i-iii. doi: 10.5588/ijtld.14.0262. Int J Tuberc Lung Dis. 2015. PMID: 25686134 Review.
Cited by
-
Enhanced Serum IgG Detection Potential Using 38KD-MPT32-MPT64, CFP10-Mtb81-EspC Fusion Protein and Lipoarabinomannan (LAM) for Human Tuberculosis.Pathogens. 2022 Dec 15;11(12):1545. doi: 10.3390/pathogens11121545. Pathogens. 2022. PMID: 36558879 Free PMC article.
References
-
- Global Tuberculosis Report 2019. [(accessed on 12 February 2021)]; Available online: https://www.who.int/teams/global-tuberculosis-programme/tb-reports.
-
- WHO XpertMTB/RIF:WHO Policy Update and Implementation Manual. [(accessed on 12 February 2021)]; Available online: http://appswhoint/iris/bitstream/handle/10665/112469/9789241506700_engpdf.
Grants and funding
LinkOut - more resources
Full Text Sources

