The Road to Low-Dose Aspirin Therapy for the Prevention of Preeclampsia Began with the Placenta
- PMID: 34209594
- PMCID: PMC8268135
- DOI: 10.3390/ijms22136985
The Road to Low-Dose Aspirin Therapy for the Prevention of Preeclampsia Began with the Placenta
Abstract
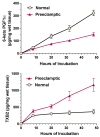
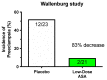
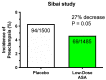
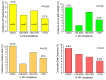
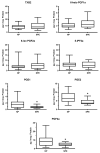
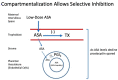
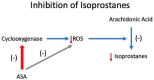
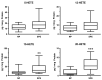
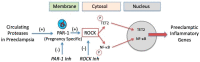
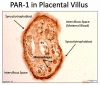
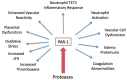
The road to low-dose aspirin therapy for the prevention of preeclampsia began in the 1980s with the discovery that there was increased thromboxane and decreased prostacyclin production in placentas of preeclamptic women. At the time, low-dose aspirin therapy was being used to prevent recurrent myocardial infarction and other thrombotic events based on its ability to selectively inhibit thromboxane synthesis without affecting prostacyclin synthesis. With the discovery that thromboxane was increased in preeclamptic women, it was reasonable to evaluate whether low-dose aspirin would be effective for preeclampsia prevention. The first clinical trials were very promising, but then two large multi-center trials dampened enthusiasm until meta-analysis studies showed aspirin was effective, but with caveats. Low-dose aspirin was most effective when started <16 weeks of gestation and at doses >100 mg/day. It was effective in reducing preterm preeclampsia, but not term preeclampsia, and patient compliance and patient weight were important variables. Despite the effectiveness of low-dose aspirin therapy in correcting the placental imbalance between thromboxane and prostacyclin and reducing oxidative stress, some aspirin-treated women still develop preeclampsia. Alterations in placental sphingolipids and hydroxyeicosatetraenoic acids not affected by aspirin, but with biologic actions that could cause preeclampsia, may explain treatment failures. Consideration should be given to aspirin's effect on neutrophils and pregnancy-specific expression of protease-activated receptor 1, as well as additional mechanisms of action to prevent preeclampsia.
Keywords: eicosanoids; isoprostanes; low-dose aspirin; neutrophils; placenta; preeclampsia; prostacyclin; protease-activated receptor 1; sphingolipids; thromboxane.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Pregnancy-specific expression of protease-activated receptor 1: a therapeutic target for prevention and treatment of preeclampsia?Am J Obstet Gynecol. 2022 Feb;226(2S):S945-S953. doi: 10.1016/j.ajog.2021.11.1367. Am J Obstet Gynecol. 2022. PMID: 35177224 Free PMC article. Review.
-
Placental Production of Eicosanoids and Sphingolipids in Women Who Developed Preeclampsia on Low-Dose Aspirin.Reprod Sci. 2020 Dec;27(12):2158-2169. doi: 10.1007/s43032-020-00234-2. Epub 2020 Jun 17. Reprod Sci. 2020. PMID: 32557282 Free PMC article.
-
Eicosanoids in preeclampsia.Prostaglandins Leukot Essent Fatty Acids. 2004 Feb;70(2):223-32. doi: 10.1016/j.plefa.2003.04.010. Prostaglandins Leukot Essent Fatty Acids. 2004. PMID: 14683695 Review.
-
Aspirin inhibits both lipid peroxides and thromboxane in preeclamptic placentas.Free Radic Biol Med. 1995 Mar;18(3):585-91. doi: 10.1016/0891-5849(94)00157-f. Free Radic Biol Med. 1995. PMID: 9101251
-
Trophoblast and placental villous core production of lipid peroxides, thromboxane, and prostacyclin in preeclampsia.J Clin Endocrinol Metab. 1995 Jun;80(6):1888-93. doi: 10.1210/jcem.80.6.7775637. J Clin Endocrinol Metab. 1995. PMID: 7775637
Cited by
-
Aspirin for preeclampsia prevention in low- and middle-income countries: mind the gaps.AJOG Glob Rep. 2024 Apr 3;4(2):100352. doi: 10.1016/j.xagr.2024.100352. eCollection 2024 May. AJOG Glob Rep. 2024. PMID: 38694484 Free PMC article.
-
Low-Dose Aspirin after ASPRE-More Questions Than Answers? Current International Approach after PE Screening in the First Trimester.Biomedicines. 2023 May 23;11(6):1495. doi: 10.3390/biomedicines11061495. Biomedicines. 2023. PMID: 37371598 Free PMC article. Review.
-
Placental Related Disorders of Pregnancy.Int J Mol Sci. 2022 Mar 24;23(7):3519. doi: 10.3390/ijms23073519. Int J Mol Sci. 2022. PMID: 35408880 Free PMC article.
-
Placental and Renal Pathways Underlying Pre-Eclampsia.Int J Mol Sci. 2024 Feb 27;25(5):2741. doi: 10.3390/ijms25052741. Int J Mol Sci. 2024. PMID: 38473987 Free PMC article. Review.
-
Risk of Thrombosis, Pregnancy Morbidity or Death in Antiphospholipid Syndrome.Front Cardiovasc Med. 2022 Mar 1;9:852777. doi: 10.3389/fcvm.2022.852777. eCollection 2022. Front Cardiovasc Med. 2022. PMID: 35299976 Free PMC article. Review.
References
-
- Moncada S., Gryglewski R.J., Bunting S., Vane J.R. A lipid peroxide inhibits the enzyme in blood vessel microsomes that generates from prostaglandin endoperoxides the substance (prostaglandin X) which prevents platelet aggregation. Prostaglandins. 1976;12:715–737. doi: 10.1016/0090-6980(76)90048-4. - DOI - PubMed
-
- Moncada S., Vane J.R. Pharmacology and endogenous roles of prostaglandin endoperoxides, thromboxane A2, and prostacyclin. Pharmacol. Rev. 1979;30:293–331. - PubMed
-
- Lewis H.D., Jr., Davis J.W., Archibald D.G., Steinke W.E., Smitherman T.C., Doherty J.E., 3rd, Schnaper H.W., LeWinter M.M., Linares E., Pouget J.M., et al. Protective effects of aspirin against acute myocardial infarction and death in men with unstable angina. Results of a Veterans Administration Cooperative Study. N. Engl. J. Med. 1983;309:396–403. doi: 10.1056/NEJM198308183090703. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

