Interrelations between Patients' Clinicopathological Characteristics and Their Association with Response to Immunotherapy in a Real-World Cohort of NSCLC Patients
- PMID: 34209601
- PMCID: PMC8268100
- DOI: 10.3390/cancers13133249
Interrelations between Patients' Clinicopathological Characteristics and Their Association with Response to Immunotherapy in a Real-World Cohort of NSCLC Patients
Abstract
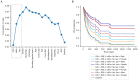
Immune checkpoint inhibitors (ICIs) have transformed non-small cell lung cancer (NSCLC) treatment. Unfortunately, only some patients benefit from these therapies. Thus, certain clinicopathological characteristics of the patients have been proposed as biomarkers of ICIs response. We assembled a retrospective cohort of 262 NSCLC patients treated with ICIs, compiled relevant clinicopathological characteristics, and studied their associations with treatment outcome using Cox proportional-hazards survival models. Additionally, we investigated the interrelations between clinicopathological features and devised a method to create a compendium associated with ICIs response by selecting those that provide non-redundant information. In multivariate analyses, ECOG performance status (hazard ratio (HR) 1.37 (95% CI 1.11 to 1.68), p < 0.005), LDH (HR 1.24 (95% CI 1.03 to 1.48), p = 0.02)) and PD-L1 negativity were associated with decreased PFS (HR 1.92 (95% CI 1.03 to 3.58), p < 0.04), whereas presentation of immune-related adverse events (irAEs) (HR 0.35 (95% CI 0.22 to 0.55, p < 0.005) or females (HR 0.52 (95% CI 0.33 to 0.80, p < 0.005) had longer progression-free survival. Additionally, numerous clinicopathological indicators were found to be interrelated. Thus, we searched for features that provide non-redundant information, and found the combination of LDH levels, irAEs, and gender to have a better association with ICIs treatment response (cross-validated c-index = 0.66). We concluded that several clinicopathological features showed prognostic value in our real-world cohort. However, some are interrelated, and compendiums of features should therefore consider these interactions. Joint assessment of LDH, irAEs, and gender may be a good prognostic compendium.
Keywords: LDH; NSCLC; biomarkers; immune checkpoint inhibitors; immune related adverse events; immunotherapy.
Conflict of interest statement
E.F. reports the following conflicts of interest: advisory role or speaker’s bureau: AbbVie, AMGEN, AstraZeneca, Bayer, Beigene, Blueprint medicines, Boehringer Ingelheim, Bristol-Meyers Squibb, CME outfitters, Eli Lilly, Glaxo smith kline, Janssen, Medscape, Medical trends, Merck KGaA, Merck Sharp & Dohme, Merck Serono, Novartis, Peervoice, Peptomyc, Pfizer, priME Oncology, Puma, Regeneron, Roche, Sanofi, Syneos health, Springer, Takeda, Touchtime. Board: Grifols, independent member. Research funding: Fundación Merck Salud, Grant for Oncology Innovation EMD Serono. C.C. and J.F. had been partially supported by Grant for Oncology EMD Serono research funding to EF. A.M.M. provided consultation, attended advisory boards and/or speaker’s bureau for the following organizations: Bristol-Myers Squibb, Lilly, F. Roche, MSD oncology, Pfizer, Boehringer Ingelheim, AstraZeneca. A.N. reports advisory role, speaker’s bureau or travel compensation: Bristol-Myers Squibb, F. Hoffmann La Roche AG, Pfizer, Boehringer Ingelheim, Oryzon Genomics, Pfizer, AstraZeneca. S.C. Bristol-Myers Squibb Recipient F, Hoffmann La Roche AG, Pfizer, Boehringer Ingelheim, MSD Oncology, Amphera. A.C. reports advisory role and/or travel compensation: Bristol-Myers Squibb Recipient, F. Hoffmann La Roche AG, Pfizer, Boehringer Ingelheim, MSD Oncology, Kyowa Kirin, Celgene, Leo Pharma, Medscape, Kern Pharma. P.I. reports advisory role and/or travel compensation: Bristol-Myers Squibb Recipient, F. Hoffmann, La Roche AG, Merck Sharp & Dohme, Boehringer Ingelheim, MSD Oncology, Rovi, Kyowa Kirin, Grunenthal Pharma S.A., Pfizer, Medscape, Kern Pharma. N.P. reports advisory role and/or travel compensation: MSD oncology, Merck Sharp & Dohme, Bristol-Myers Squibb Recipient, F. Hoffmann La Roche AG, Pfizer, Boehringer Ingelheim, Grunenthal Pharma S.A Kern Pharma. All remaining authors have declared no conflict of interest.
Figures

References
-
- Rizvi H., Sanchez-Vega F., La K., Chatila W., Jonsson P., Halpenny D., Plodkowski A., Long N., Sauter J.L., Rekhtman N., et al. Molecular determinants of response to anti-programmed cell death (PD)-1 and anti-programmed death-ligand 1 (PD-L1) blockade in patients with non-small-cell lung cancer profiled with targeted next-generation sequencing. J. Clin. Oncol. 2018;36:633–641. doi: 10.1200/JCO.2017.75.3384. - DOI - PMC - PubMed
-
- Hellmann M.D., Nathanson T., Rizvi H., Creelan B.C., Sanchez-Vega F., Ahuja A., Ni A., Novik J.B., Mangarin L.M.B., Abu-Akeel M., et al. Genomic Features of Response to Combination Immunotherapy in Patients with Advanced Non-Small-Cell Lung Cancer. Cancer Cell. 2018;33:843–852.e4. doi: 10.1016/j.ccell.2018.03.018. - DOI - PMC - PubMed
-
- Frigola J., Navarro A., Carbonell C., Callejo A., Iranzo P., Cedrés S., Martinez-Marti A., Pardo N., Saoudi-Gonzalez N., Martinez D., et al. Molecular profiling of long-term responders to immune checkpoint inhibitors in advanced non-small cell lung cancer. Mol. Oncol. 2020:1–14. doi: 10.1002/1878-0261.12891. - DOI - PMC - PubMed
-
- Bagley S.J., Kothari S., Aggarwal C., Bauml J.M., Alley E.W., Evans T.L., Kosteva J.A., Ciunci C.A., Gabriel P.E., Thompson J.C., et al. Pretreatment neutrophil-to-lymphocyte ratio as a marker of outcomes in nivolumab-treated patients with advanced non-small-cell lung cancer. Lung Cancer. 2017;106:1–7. doi: 10.1016/j.lungcan.2017.01.013. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

