Melatonin Ameliorates the Toxicity Induced by Deoxynivalenol in Murine Ovary Granulosa Cells by Antioxidative and Anti-Inflammatory Effects
- PMID: 34209652
- PMCID: PMC8300713
- DOI: 10.3390/antiox10071045
Melatonin Ameliorates the Toxicity Induced by Deoxynivalenol in Murine Ovary Granulosa Cells by Antioxidative and Anti-Inflammatory Effects
Abstract
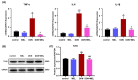
Melatonin is an important endogenous hormone that shows antioxidant functions and pleiotropic effects, playing a crucial role in animal reproduction. Ovary granulosa cells (GCs) surround the oocyte, which play an important role in regulating oocytes development. Deoxynivalenol (DON) is a common fusarium mycotoxin contaminant of feedstuff and food, posing a serious threat to human and animal reproductive systems. Herein, murine ovary GCs were studied as a reproduction cell model, aimed to assess the protective effect of melatonin on DON-induced toxicity in murine ovary GCs. The results showed that DON adversely affected the viability and growth of murine ovary GCs and increased the apoptosis rate, while melatonin administration ameliorated these toxic effects. We further reveal that DON exposure increased the intracellular reactive oxygen species level, reduced the mitochondrial membrane potential and ATP, and upregulated Tnfα (tumor necrosis factor α), Il6 (interleukin 6), and Il1β (interleukin 1 β) gene expression. Moreover, DON exposure downregulated reproductive hormone gene expression and significantly increased nuclear factor kappa B (p65) activation and mitogen-activated protein kinase phosphorylation. Melatonin treatment attenuated all these effects, suggesting that melatonin protects GCs from the adverse effects of DON by ameliorating oxidative stress, mitochondrial dysfunction, and inflammation. Overall, these results reveal the mechanisms of DON and melatonin in GCs and provide a theoretical basis for melatonin as a drug to improve mycotoxin contamination.
Keywords: anti inflammatory; antioxidation; apoptosis; deoxynivalenol; melatonin; ovary granulosa cells.
Conflict of interest statement
The authors declare no competing financial interest.
Figures






References
-
- Robinson J.W., Zhang M., Shuhaibar L.C., Norris R.P., Geerts A., Wunder F., Eppig J.J., Potter L.R., Jaffe L.A. Luteinizing hormone reduces the activity of the NPR2 guanylyl cyclase in mouse ovarian follicles, contributing to the cyclic GMP decrease that promotes resumption of meiosis in oocytes. Dev. Biol. 2012;366:308–316. doi: 10.1016/j.ydbio.2012.04.019. - DOI - PMC - PubMed
-
- Cai L., Sun A., Li H., Tsinkgou A., Yu J., Ying S., Chen Z., Shi Z. Molecular mechanisms of enhancing porcine granulosa cell proliferation and function by treatment in vitro with anti-inhibin alpha subunit antibody. Reprod Biol Endocrinol. 2015;13:26. doi: 10.1186/s12958-015-0022-3. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

