Meroterpenoids: A Comprehensive Update Insight on Structural Diversity and Biology
- PMID: 34209734
- PMCID: PMC8301922
- DOI: 10.3390/biom11070957
Meroterpenoids: A Comprehensive Update Insight on Structural Diversity and Biology
Abstract
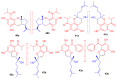
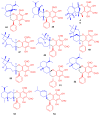
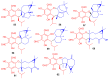
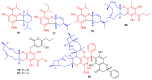
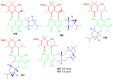
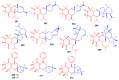
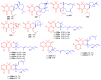
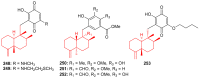
Meroterpenoids are secondary metabolites formed due to mixed biosynthetic pathways which are produced in part from a terpenoid co-substrate. These mixed biosynthetically hybrid compounds are widely produced by bacteria, algae, plants, and animals. Notably amazing chemical diversity is generated among meroterpenoids via a combination of terpenoid scaffolds with polyketides, alkaloids, phenols, and amino acids. This review deals with the isolation, chemical diversity, and biological effects of 452 new meroterpenoids reported from natural sources from January 2016 to December 2020. Most of the meroterpenoids possess antimicrobial, cytotoxic, antioxidant, anti-inflammatory, antiviral, enzyme inhibitory, and immunosupressive effects.
Keywords: anti-inflammatory; anticancer; antidiabetic; antioxidant; meroterpenoids; structural diversity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

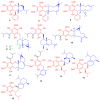
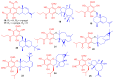
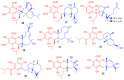
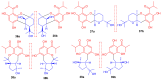

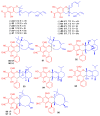

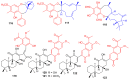
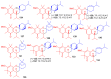
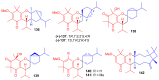
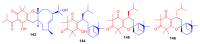
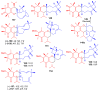
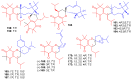

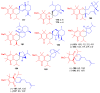





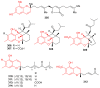

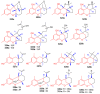
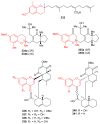
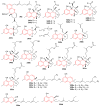


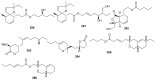
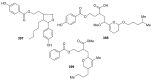

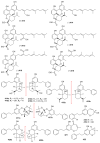
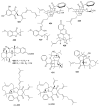
References
-
- Brahmachari G. Discovery and Development of Neuroprotective Agents from Natural Products: An Overview. In: Brahmachari G., editor. Discovery and Development of Neuroprotective Agents from Natural Products. 1st ed. Elsevier; Amsterdam, The Netherlands: 2018. pp. 1–7.
-
- Swargiary A. Recent trends in traditionally used medicinal plants and drug discovery. Asian J. Pharm. Pharmacol. 2017;3:111–120.
-
- Annang F., Genilloud O., Vicente F. Contribution of Natural Products to Drug Discovery in Tropical Diseases. In: Müller S., Cerdan R., Radulescu O., editors. Comprehensive Analysis of Parasite Biology, From Metabolism to Drug Discovery. Wiley-VCH; Weheim, Germany: 2016. pp. 75–104.
-
- David B., Wolfender J.L., Dias D.A. The pharmaceutical industry and natural products, historical status and new trends. Phytochem. Rev. 2015;14:299–315. doi: 10.1007/s11101-014-9367-z. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

