Iron Released after Cryo-Thermal Therapy Induced M1 Macrophage Polarization, Promoting the Differentiation of CD4+ T Cells into CTLs
- PMID: 34209797
- PMCID: PMC8268875
- DOI: 10.3390/ijms22137010
Iron Released after Cryo-Thermal Therapy Induced M1 Macrophage Polarization, Promoting the Differentiation of CD4+ T Cells into CTLs
Abstract
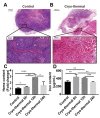
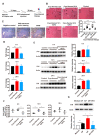
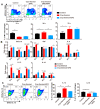
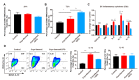
Macrophages play critical roles in both innate and adaptive immunity and are known for their high plasticity in response to various external signals. Macrophages are involved in regulating systematic iron homeostasis and they sequester iron by phagocytotic activity, which triggers M1 macrophage polarization and typically exerts antitumor effects. We previously developed a novel cryo-thermal therapy that can induce the mass release of tumor antigens and damage-associated molecular patterns (DAMPs), promoting M1 macrophage polarization. However, that study did not examine whether iron released after cryo-thermal therapy induced M1 macrophage polarization; this question still needed to be addressed. We hypothesized that cryo-thermal therapy would cause the release of a large quantity of iron to augment M1 macrophage polarization due to the disruption of tumor cells and blood vessels, which would further enhance antitumor immunity. In this study, we investigated iron released in primary tumors, the level of iron in splenic macrophages after cryo-thermal therapy and the effect of iron on macrophage polarization and CD4+ T cell differentiation in metastatic 4T1 murine mammary carcinoma. We found that a large amount of iron was released after cryo-thermal therapy and could be taken up by splenic macrophages, which further promoted M1 macrophage polarization by inhibiting ERK phosphorylation. Moreover, iron promoted DC maturation, which was possibly mediated by iron-induced M1 macrophages. In addition, iron-induced M1 macrophages and mature DCs promoted the differentiation of CD4+ T cells into the CD4 cytolytic T lymphocytes (CTL) subset and inhibited differentiation into Th2 and Th17 cells. This study explains the role of iron in cryo-thermal therapy-induced antitumor immunity from a new perspective.
Keywords: CD4 CTL; CD4+ T cell differentiation; M1 macrophages; cryo-thermal therapy; iron.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Cryo-thermal therapy inducing MI macrophage polarization created CXCL10 and IL-6-rich pro-inflammatory environment for CD4+ T cell-mediated anti-tumor immunity.Int J Hyperthermia. 2019;36(1):408-420. doi: 10.1080/02656736.2019.1579373. Epub 2019 Mar 20. Int J Hyperthermia. 2019. PMID: 30892102
-
A role of eosinophils in mediating the anti-tumour effect of cryo-thermal treatment.Sci Rep. 2019 Sep 13;9(1):13214. doi: 10.1038/s41598-019-49734-5. Sci Rep. 2019. PMID: 31519961 Free PMC article.
-
Tumor-related HSP70 released after cryo-thermal therapy targeted innate immune initiation in the antitumor immune response.Int J Hyperthermia. 2020;37(1):843-853. doi: 10.1080/02656736.2020.1788173. Int J Hyperthermia. 2020. PMID: 32654540
-
Diverse macrophages polarization in tumor microenvironment.Arch Pharm Res. 2016 Nov;39(11):1588-1596. doi: 10.1007/s12272-016-0820-y. Epub 2016 Aug 25. Arch Pharm Res. 2016. PMID: 27562774 Review.
-
Macrophage Polarization: Different Gene Signatures in M1(LPS+) vs. Classically and M2(LPS-) vs. Alternatively Activated Macrophages.Front Immunol. 2019 May 24;10:1084. doi: 10.3389/fimmu.2019.01084. eCollection 2019. Front Immunol. 2019. PMID: 31178859 Free PMC article. Review.
Cited by
-
Targeting and activation of macrophages in leishmaniasis. A focus on iron oxide nanoparticles.Front Immunol. 2024 Aug 15;15:1437430. doi: 10.3389/fimmu.2024.1437430. eCollection 2024. Front Immunol. 2024. PMID: 39211053 Free PMC article. Review.
-
A novel HVEM-Fc recombinant protein for lung cancer immunotherapy.J Exp Clin Cancer Res. 2025 Feb 20;44(1):62. doi: 10.1186/s13046-025-03324-8. J Exp Clin Cancer Res. 2025. PMID: 39979981 Free PMC article.
-
Applications of Magnetite Nanoparticles in Cancer Immunotherapies: Present Hallmarks and Future Perspectives.Front Immunol. 2021 Oct 4;12:701485. doi: 10.3389/fimmu.2021.701485. eCollection 2021. Front Immunol. 2021. PMID: 34675914 Free PMC article. Review.
-
Activated NK cells reprogram MDSCs via NKG2D-NKG2DL and IFN-γ to modulate antitumor T-cell response after cryo-thermal therapy.J Immunother Cancer. 2022 Dec;10(12):e005769. doi: 10.1136/jitc-2022-005769. J Immunother Cancer. 2022. PMID: 36521929 Free PMC article.
-
Role of neutrophil extracellular traps in inflammatory evolution in severe acute pancreatitis.Chin Med J (Engl). 2022 Dec 5;135(23):2773-2784. doi: 10.1097/CM9.0000000000002359. Chin Med J (Engl). 2022. PMID: 36729096 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

