Landscape of Preterm Birth Therapeutics and a Path Forward
- PMID: 34209869
- PMCID: PMC8268657
- DOI: 10.3390/jcm10132912
Landscape of Preterm Birth Therapeutics and a Path Forward
Abstract
Preterm birth (PTB) remains the leading cause of infant morbidity and mortality. Despite 50 years of research, therapeutic options are limited and many lack clear efficacy. Tocolytic agents are drugs that briefly delay PTB, typically to allow antenatal corticosteroid administration for accelerating fetal lung maturity or to transfer patients to high-level care facilities. Globally, there is an unmet need for better tocolytic agents, particularly in low- and middle-income countries. Although most tocolytics, such as betamimetics and indomethacin, suppress downstream mediators of the parturition pathway, newer therapeutics are being designed to selectively target inflammatory checkpoints with the goal of providing broader and more effective tocolysis. However, the relatively small market for new PTB therapeutics and formidable regulatory hurdles have led to minimal pharmaceutical interest and a stagnant drug pipeline. In this review, we present the current landscape of PTB therapeutics, assessing the history of drug development, mechanisms of action, adverse effects, and the updated literature on drug efficacy. We also review the regulatory hurdles and other obstacles impairing novel tocolytic development. Ultimately, we present possible steps to expedite drug development and meet the growing need for effective preterm birth therapeutics.
Keywords: fetus; neonate; pregnancy; prematurity; preterm birth; preterm labor; progesterone; therapeutic; tocolytic.
Conflict of interest statement
Alisa Kachikis is on a Pfizer and GlaxoSmithKline Advisory Board for Immunizations, which is unrelated to the content of this manuscript. David Olson and Sylvain Chemtob are founders of Maternica Therapeutics, Inc., and David Olson is founder and CEO of Livmor Therapeutics, Inc. David Olson and Sylvain Chemtob were the primary authors of the following section(s) of this manuscript: Section 12. Jonathan W. Paul and Roger Smith hold patents in the United States, Europe, and Australia related to reproductive medical applications of uterine-targeted nanoparticles. Jonathan Paul and Roger Smith were the primary authors of the following section(s) of this manuscript: Section 13. The remaining authors report no conflict of interest.
Figures
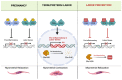

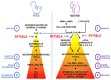
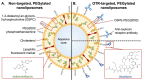
References
-
- Blencowe H., Cousens S., Oestergaard M.Z., Chou D., Moller A.B., Narwal R., Adler A., Vera Garcia C., Rohde S., Say L., et al. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: A systematic analysis and implications. Lancet. 2012;379:2162–2172. doi: 10.1016/S0140-6736(12)60820-4. - DOI - PubMed
-
- Suman V., Luther E.E. StatPearls. StatPearls Publishing; Treasure Island, FL, USA: 2021. Preterm Labor. - PubMed
Publication types
Grants and funding
- FDN-143262/CAPMC/ CIHR/Canada
- R01 AI145890/AI/NIAID NIH HHS/United States
- 168858/CAPMC/ CIHR/Canada
- AI145890/National Institute of Allergy and Infectious Diseases
- U42 OD011123/OD/NIH HHS/United States
- MOD-6-FY17-647/March of Dimes Foundation
- AI143265/National Institute of Allergy and Infectious Diseases
- HD098713/National Institute of Allergy and Infectious Diseases
- 1013759/Burroughs Wellcome Fund
- P51 OD010425/OD/NIH HHS/United States
- R01 HD098713/HD/NICHD NIH HHS/United States
- AI133976/National Institute of Allergy and Infectious Diseases
- R01 AI133976/AI/NIAID NIH HHS/United States
- APP1113847/National Health and Medical Research Council
- APP1162684/National Health and Medical Research Council
- HD001264/National Institute of Allergy and Infectious Diseases
LinkOut - more resources
Full Text Sources

