Evidence for the Role of Mitochondrial DNA Release in the Inflammatory Response in Neurological Disorders
- PMID: 34209978
- PMCID: PMC8268735
- DOI: 10.3390/ijms22137030
Evidence for the Role of Mitochondrial DNA Release in the Inflammatory Response in Neurological Disorders
Abstract
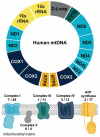
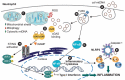
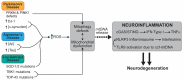
Mitochondria are regarded as the metabolic centers of cells and are integral in many other cell processes, including the immune response. Each mitochondrion contains numerous copies of mitochondrial DNA (mtDNA), a small, circular, and bacterial-like DNA. In response to cellular damage or stress, mtDNA can be released from the mitochondrion and trigger immune and inflammatory responses. mtDNA release into the cytosol or bloodstream can occur as a response to hypoxia, sepsis, traumatic injury, excitatory cytotoxicity, or drastic mitochondrial membrane potential changes, some of which are hallmarks of neurodegenerative and mood disorders. Released mtDNA can mediate inflammatory responses observed in many neurological and mood disorders by driving the expression of inflammatory cytokines and the interferon response system. The current understanding of the role of mtDNA release in affective mood disorders and neurodegenerative diseases will be discussed.
Keywords: inflammation; mitochondria; mitochondrial DNA (mtDNA); neurodegenerative disease; neuropsychiatric disorder; reactive oxygen species (ROS).
Conflict of interest statement
P.D.R. is on the board of the Ottagan Addiction Recovery program. G.E.M. and K.E.D.-R. declare no conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

