Epstein-Barr Virus DNA Exacerbates Colitis Symptoms in a Mouse Model of Inflammatory Bowel Disease
- PMID: 34210024
- PMCID: PMC8310145
- DOI: 10.3390/v13071272
Epstein-Barr Virus DNA Exacerbates Colitis Symptoms in a Mouse Model of Inflammatory Bowel Disease
Abstract
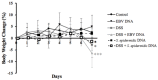
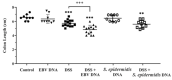
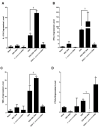
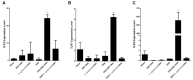
Infection with EBV has been associated with various inflammatory disorders including inflammatory bowel diseases (IBD). Contribution of this virus to intestinal disease processes has not been assessed. We previously detected that EBV DNA triggers proinflammatory responses via the activation of endosomal Toll-like receptor (TLR) signaling. Hence, to examine the colitogenic potential of EBV DNA, we used the dextran sodium sulfate (DSS) mouse colitis model. C57BL/6J mice received either DSS-containing or regular drinking water. Mice were then administered EBV DNA by rectal gavage. Administration of EBV DNA to the DSS-fed mice aggravated colonic disease activity as well as increased the damage to the colon histologic architecture. Moreover, we observed enhanced expression of IL-17A, IFNγ and TNFα in colon tissues from the colitis mice (DSS-treated) given the EBV DNA compared to the other groups. This group also had a marked decrease in expression of the CTLA4 immunoregulatory marker. On the other hand, we observed enhanced expression of endosomal TLRs in colon tissues from the EBV DNA-treated colitis mice. These findings indicate that EBV DNA exacerbates proinflammatory responses in colitis. The ubiquity of EBV in the population indicates that possible similar responses may be of pertinence in a relevant proportion of IBD patients.
Keywords: Epstein–Barr virus; IL-17A; Toll-like Receptors; inflammatory bowel disease.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

