Near-Infrared-Triggered Upconverting Nanoparticles for Biomedicine Applications
- PMID: 34210059
- PMCID: PMC8301434
- DOI: 10.3390/biomedicines9070756
Near-Infrared-Triggered Upconverting Nanoparticles for Biomedicine Applications
Abstract
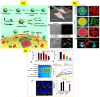
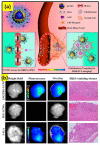
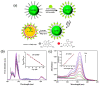
Due to the unique properties of lanthanide-doped upconverting nanoparticles (UCNP) under near-infrared (NIR) light, the last decade has shown a sharp progress in their biomedicine applications. Advances in the techniques for polymer, dye, and bio-molecule conjugation on the surface of the nanoparticles has further expanded their dynamic opportunities for optogenetics, oncotherapy and bioimaging. In this account, considering the primary benefits such as the absence of photobleaching, photoblinking, and autofluorescence of UCNPs not only facilitate the construction of accurate, sensitive and multifunctional nanoprobes, but also improve therapeutic and diagnostic results. We introduce, with the basic knowledge of upconversion, unique properties of UCNPs and the mechanisms involved in photon upconversion and discuss how UCNPs can be implemented in biological practices. In this focused review, we categorize the applications of UCNP-based various strategies into the following domains: neuromodulation, immunotherapy, drug delivery, photodynamic and photothermal therapy, bioimaging and biosensing. Herein, we also discuss the current emerging bioapplications with cutting edge nano-/biointerfacing of UCNPs. Finally, this review provides concluding remarks on future opportunities and challenges on clinical translation of UCNPs-based nanotechnology research.
Keywords: bioimaging; biomedicine; biosensors; nanoparticles; oncotherapy; optogenetics; upconversion luminescence.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

