Evidence for antibody as a protective correlate for COVID-19 vaccines
- PMID: 34210573
- PMCID: PMC8142841
- DOI: 10.1016/j.vaccine.2021.05.063
Evidence for antibody as a protective correlate for COVID-19 vaccines
Abstract
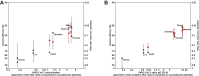
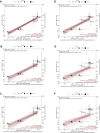
A correlate of protection (CoP) is urgently needed to expedite development of additional COVID-19 vaccines to meet unprecedented global demand. To assess whether antibody titers may reasonably predict efficacy and serve as the basis of a CoP, we evaluated the relationship between efficacy and in vitro neutralizing and binding antibodies of 7 vaccines for which sufficient data have been generated. Once calibrated to titers of human convalescent sera reported in each study, a robust correlation was seen between neutralizing titer and efficacy (ρ = 0.79) and binding antibody titer and efficacy (ρ = 0.93), despite geographically diverse study populations subject to different forces of infection and circulating variants, and use of different endpoints, assays, convalescent sera panels and manufacturing platforms. Together with evidence from natural history studies and animal models, these results support the use of post-immunization antibody titers as the basis for establishing a correlate of protection for COVID-19 vaccines.
Keywords: COVID-19; Correlate of protection; SARS-CoV-2; Vaccine.
Copyright © 2021 The Authors. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Dr. Plotkin consults for Janssen and Moderna; Dr. Siber reports personal fees from Clover Biopharmaceuticals, other from COVAXX, personal fees from CanSino, personal fees from CureVac, personal fees from Valneva, personal fees and other from Affinivax, outside the submitted work; Dr. Gilbert reports grants and non-financial support from SanofiPasteur, outside the submitted work; Dr. Ambrosino reports personal fees from COVAXX, personal fees from Clover Biopharmaceuticals, outside the submitted work.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

