Circulating HPV DNA as a Marker for Early Detection of Relapse in Patients with Cervical Cancer
- PMID: 34210686
- PMCID: PMC9401545
- DOI: 10.1158/1078-0432.CCR-21-0625
Circulating HPV DNA as a Marker for Early Detection of Relapse in Patients with Cervical Cancer
Abstract
Purpose: Almost all cervical cancers are caused by human papillomavirus (HPV) and patients with advanced stage are at high risk for relapse. Circulating HPV DNA (HPV ctDNA) may serve as a residual tumor marker at the end of chemoradiation or to predict relapse during the follow-up period.
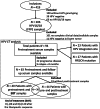
Experimental design: We analyzed serum samples from 94 HPV16- or HPV18-related CCs from the BioRAIDs prospective cohort. Samples were collected before and after treatment and during an 18-month follow-up period. Using digital droplet PCR (ddPCR), we assessed the relevance of circulating HPV E7 gene as a marker for residual disease compared to HPV integration site and PIK3CA mutations. Finally, the prognostic impact of circulating HPV E7 gene was assessed with its prediction value of relapse.
Results: HPV E7 gene was the most sensitive tumor marker, superior to both HPV integration sites and PIK3CA mutations in serum. Circulating HPV DNA (HPV ctDNA) was detected in 63% (59/94) of patients, before treatment. HPV ctDNA detection in serum sample was associated with high FIGO stage (P = 0.02) and para-aortic lymph node involvement (P = 0.01). The level of HPV ctDNA was positively correlated with HPV copy number in the tumor (R = 0.39, P < 0.001). Complete clearance of HPV ctDNA by the end of treatment was significantly associated with a longer PFS (P < 0.0001). Patients with persistent HPV ctDNA in serum relapsed with a median time of 10 months (range, 2-15) from HPV ctDNA detection.
Conclusions: HPV ctDNA detection is a useful marker to predict relapse in cervical cancer.See related commentary by Wentzensen and Clarke, p. 5733.
©2021 The Authors; Published by the American Association for Cancer Research.
Figures


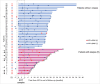
 ) and blue (
) and blue ( ) correspond to HPV-positive and HPV-negative serum samples, respectively. Serum samples in dashed area correspond to EOT samples. Patients with HPV ctDNA in their EOT samples are indicated by a black arrow. Diagnosis of relapse corresponds to the end of the line. S1, serum sample before treatment; R, relapse.
) correspond to HPV-positive and HPV-negative serum samples, respectively. Serum samples in dashed area correspond to EOT samples. Patients with HPV ctDNA in their EOT samples are indicated by a black arrow. Diagnosis of relapse corresponds to the end of the line. S1, serum sample before treatment; R, relapse.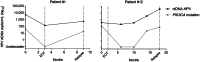
Comment in
-
Liquid Biopsy for Cancer Detection: Clinical and Epidemiologic Considerations.Clin Cancer Res. 2021 Nov 1;27(21):5733-5735. doi: 10.1158/1078-0432.CCR-21-2426. Epub 2021 Aug 30. Clin Cancer Res. 2021. PMID: 34462288
References
-
- Forman D, de Martel C, Lacey CJ, Soerjomataram I, Lortet-Tieulent J, Bruni L, et al. Global burden of human papillomavirus and related diseases. Vaccine 2012;30:F12–23. - PubMed
-
- Keys HM, Bundy BN, Stehman FB, Okagaki T, Gallup DG, Burnett AF, et al. Radiation therapy with and without extrafascial hysterectomy for bulky stage IB cervical carcinoma: a randomized trial of the Gynecologic Oncology Group. Gynecol Oncol 2003;89:343–53. - PubMed
-
- Marth C, Landoni F, Mahner S, McCormack M, Gonzalez-Martin A, Colombo N, et al. Cervical cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2017;28:iv72–83. - PubMed
-
- Kim JY, Byun SJ, Kim YS, Nam JH. Disease courses in patients with residual tumor following concurrent chemoradiotherapy for locally advanced cervical cancer. Gynecol Oncol 2017;144:34–9. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

