Association between ibrutinib treatment and hypertension
- PMID: 34210750
- PMCID: PMC9809112
- DOI: 10.1136/heartjnl-2021-319110
Association between ibrutinib treatment and hypertension
Abstract
Background: Ibrutinib is a tyrosine kinase inhibitor most commonly associated with atrial fibrillation. However, additional cardiotoxicities have been identified, including accelerated hypertension. The incidence and risk factors of new or worsening hypertension following ibrutinib treatment are not as well known.
Methods: We conducted a retrospective study of 144 patients diagnosed with B cell malignancies treated with ibrutinib (n=93) versus conventional chemoimmunotherapy (n=51) and evaluated their effects on blood pressure at 1, 2, 3 and 6 months after treatment initiation. Descriptive statistics were used to compare baseline characteristics for each treatment group. Fisher's exact test was used to identify covariates significantly associated with the development of hypertension. Repeated measures analyses were conducted to analyse longitudinal blood pressure changes.
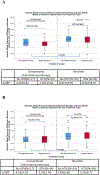
Results: Both treatments had similar prevalence of baseline hypertension at 63.4% and 66.7%, respectively. There were no differences between treatments by age, sex and baseline cardiac comorbidities. Both systolic and diastolic blood pressure significantly increased over time with ibrutinib compared with baseline, whereas conventional chemoimmunotherapy was not associated with significant changes in blood pressure. Baseline hypertensive status did not affect the degree of blood pressure change over time. A significant increase in systolic blood pressure (defined as more than 10 mm Hg) was noted for ibrutinib (36.6%) compared with conventional chemoimmunotherapy (7.9%) at 1 month after treatment initiation. Despite being hypertensive at follow-up, 61.2% of patients who were treated with ibrutinib did not receive adequate blood pressure management (increase or addition of blood pressure medications). Within the ibrutinib group, of patients who developed more than 20 mm Hg increase in systolic blood pressure, only 52.9% had hypertension management changes.
Conclusions: Ibrutinib is associated with the development of hypertension and worsening of blood pressure. Cardiologists and oncologists must be aware of this cardiotoxicity to allow timely management of blood pressure elevations.
Keywords: drug monitoring; health care; hypertension; outcome assessment.
© Author(s) (or their employer(s)) 2022. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: MF is advisor/consultant for Takeda and Abbott and received research grant from Medtronic. JP-I is investigator for Novartis and Ariad; consultant/advisor for Novartis, Bristol-Myers Squibb and Ariad; and received consulting/speakers bureau fees from Janssen and Pharmacyclics. The rest of the authors have nothing to disclose.
Figures

References
-
- Dreyling M, Jurczak W, Jerkeman M, et al. Ibrutinib versus temsirolimus in patients with relapsed or refractory mantle-cell lymphoma: an international, randomised, open-label, phase 3 study. Lancet 2016;387:770–8. - PubMed
-
- Hallek M, Fischer K, Fingerle-Rowson G, et al. Addition of rituximab to fludarabine and cyclophosphamide in patients with chronic lymphocytic leukaemia: a randomised, open-label, phase 3 trial. Lancet 2010;376:1164–74. - PubMed
-
- Knauf WU, Lissichkov T, Aldaoud A, et al. Phase III randomized study of bendamustine compared with chlorambucil in previously untreated patients with chronic lymphocytic leukemia. J Clin Oncol 2009;27:4378–84. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
