CD8+ T cells specific for conserved coronavirus epitopes correlate with milder disease in COVID-19 patients
- PMID: 34210785
- PMCID: PMC8975171
- DOI: 10.1126/sciimmunol.abg5669
CD8+ T cells specific for conserved coronavirus epitopes correlate with milder disease in COVID-19 patients
Abstract
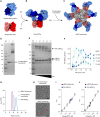
A central feature of the SARS-CoV-2 pandemic is that some individuals become severely ill or die, whereas others have only a mild disease course or are asymptomatic. Here we report development of an improved multimeric αβ T cell staining reagent platform, with each maxi-ferritin "spheromer" displaying 12 peptide-MHC complexes. Spheromers stain specific T cells more efficiently than peptide-MHC tetramers and capture a broader portion of the sequence repertoire for a given peptide-MHC. Analyzing the response in unexposed individuals, we find that T cells recognizing peptides conserved amongst coronaviruses are more abundant and tend to have a "memory" phenotype, compared to those unique to SARS-CoV-2. Significantly, CD8+ T cells with these conserved specificities are much more abundant in COVID-19 patients with mild disease versus those with a more severe illness, suggesting a protective role.
Copyright © 2021, American Association for the Advancement of Science.
Figures







References
-
- Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., Cheng Z., Yu T., Xia J., Wei Y., Wu W., Xie X., Yin W., Li H., Liu M., Xiao Y., Gao H., Guo L., Xie J., Wang G., Jiang R., Gao Z., Jin Q., Wang J., Cao B., Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 395, 497–506 (2020). - PMC - PubMed
-
- Krammer F., SARS-CoV-2 vaccines in development. Nature 586, 516–527 (2020). - PubMed
-
- Crawford K. H. D., Dingens A. S., Eguia R., Wolf C. R., Wilcox N., Logue J. K., Shuey K., Casto A. M., Fiala B., Wrenn S., Pettie D., King N. P., Greninger A. L., Chu H. Y., Bloom J. D., Dynamics of neutralizing antibody titers in the months after SARS-CoV-2 infection. J. Infect. Dis. jiaa618 (2020). - PMC - PubMed
-
- Seow J., Graham C., Merrick B., Acors S., Pickering S., Steel K. J. A., Hemmings O., O’Byrne A., Kouphou N., Galao R. P., Betancor G., Wilson H. D., Signell A. W., Winstone H., Kerridge C., Huettner I., Jimenez-Guardeño J. M., Lista M. J., Temperton N., Snell L. B., Bisnauthsing K., Moore A., Green A., Martinez L., Stokes B., Honey J., Izquierdo-Barras A., Arbane G., Patel A., Tan M. K. I., O’Connell L., O’Hara G., Mahon E. M., Douthwaite S., Nebbia G., Batra R., Martinez-Nunez R., Shankar-Hari M., Edgeworth J. D., Neil S. J. D., Malim M. H., Doores K. J., Longitudinal observation and decline of neutralizing antibody responses in the three months following SARS-CoV-2 infection in humans. Nat. Microbiol. 5, 1598–1607 (2020). - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

