SARS-CoV-2 immune evasion by the B.1.427/B.1.429 variant of concern
- PMID: 34210893
- PMCID: PMC9835956
- DOI: 10.1126/science.abi7994
SARS-CoV-2 immune evasion by the B.1.427/B.1.429 variant of concern
Abstract
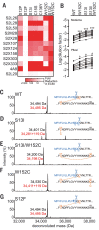
A novel variant of concern (VOC) named CAL.20C (B.1.427/B.1.429), which was originally detected in California, carries spike glycoprotein mutations S13I in the signal peptide, W152C in the N-terminal domain (NTD), and L452R in the receptor-binding domain (RBD). Plasma from individuals vaccinated with a Wuhan-1 isolate-based messenger RNA vaccine or from convalescent individuals exhibited neutralizing titers that were reduced 2- to 3.5-fold against the B.1.427/B.1.429 variant relative to wild-type pseudoviruses. The L452R mutation reduced neutralizing activity in 14 of 34 RBD-specific monoclonal antibodies (mAbs). The S13I and W152C mutations resulted in total loss of neutralization for 10 of 10 NTD-specific mAbs because the NTD antigenic supersite was remodeled by a shift of the signal peptide cleavage site and the formation of a new disulfide bond, as revealed by mass spectrometry and structural studies.
Copyright © 2021, American Association for the Advancement of Science.
Figures


Update of
-
SARS-CoV-2 immune evasion by variant B.1.427/B.1.429.bioRxiv [Preprint]. 2021 Apr 1:2021.03.31.437925. doi: 10.1101/2021.03.31.437925. bioRxiv. 2021. Update in: Science. 2021 Aug 6;373(6555):648-654. doi: 10.1126/science.abi7994. PMID: 33821281 Free PMC article. Updated. Preprint.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

