Inflammasomes as therapeutic targets in human diseases
- PMID: 34210954
- PMCID: PMC8249422
- DOI: 10.1038/s41392-021-00650-z
Inflammasomes as therapeutic targets in human diseases
Abstract
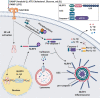
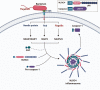
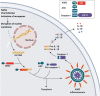
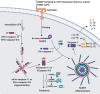
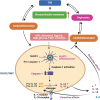
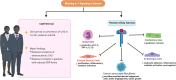
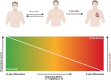
Inflammasomes are protein complexes of the innate immune system that initiate inflammation in response to either exogenous pathogens or endogenous danger signals. Inflammasome multiprotein complexes are composed of three parts: a sensor protein, an adaptor, and pro-caspase-1. Activation of the inflammasome leads to the activation of caspase-1, which cleaves pro-inflammatory cytokines such as IL-1β and IL-18, leading to pyroptosis. Effectors of the inflammasome not only provide protection against infectious pathogens, but also mediate control over sterile insults. Aberrant inflammasome signaling has been implicated in the development of cardiovascular and metabolic diseases, cancer, and neurodegenerative disorders. Here, we review the role of the inflammasome as a double-edged sword in various diseases, and the outcomes can be either good or bad depending on the disease, as well as the genetic background. We highlight inflammasome memory and the two-shot activation process. We also propose the M- and N-type inflammation model, and discuss how the inflammasome pathway may be targeted for the development of novel therapy.
Conflict of interest statement
The authors declare no competing interests.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

