Canagliflozin protects against sepsis capillary leak syndrome by activating endothelial α1AMPK
- PMID: 34211080
- PMCID: PMC8249425
- DOI: 10.1038/s41598-021-93156-1
Canagliflozin protects against sepsis capillary leak syndrome by activating endothelial α1AMPK
Abstract
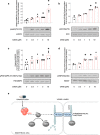
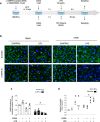
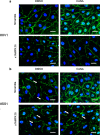
Sepsis capillary leak syndrome (SCLS) is an independent prognostic factor for poor sepsis outcome. We previously demonstrated that α1AMP-activated protein kinase (α1AMPK) prevents sepsis-induced vascular hyperpermeability by mechanisms involving VE-cadherin (VE-Cad) stabilization and activation of p38 mitogen activated protein kinase/heat shock protein of 27 kDa (p38MAPK/HSP27) pathway. Canagliflozin, a sodium-glucose co-transporter 2 inhibitor, has recently been proven to activate AMPK in endothelial cells. Therefore, we hypothesized that canagliflozin could be of therapeutic potential in patients suffering from SCLS. We herein report that canagliflozin, used at clinically relevant concentrations, counteracts lipopolysaccharide-induced vascular hyperpermeability and albumin leakage in wild-type, but not in endothelial-specific α1AMPK-knockout mice. In vitro, canagliflozin was demonstrated to activate α1AMPK/p38MAPK/HSP27 pathway and to preserve VE-Cad's integrity in human endothelial cells exposed to human septic plasma. In conclusion, our data demonstrate that canagliflozin protects against SCLS via an α1AMPK-dependent pathway, and lead us to consider novel therapeutic perspectives for this drug in SCLS.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

