Quantitative flux analysis in mammals
- PMID: 34211182
- PMCID: PMC9289955
- DOI: 10.1038/s42255-021-00419-2
Quantitative flux analysis in mammals
Abstract
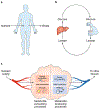
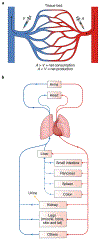
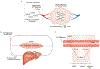
Altered metabolic activity contributes to the pathogenesis of a number of diseases, including diabetes, heart failure, cancer, fibrosis and neurodegeneration. These diseases, and organismal metabolism more generally, are only partially recapitulated by cell culture models. Accordingly, it is important to measure metabolism in vivo. Over the past century, researchers studying glucose homeostasis have developed strategies for the measurement of tissue-specific and whole-body metabolic activity (pathway fluxes). The power of these strategies has been augmented by recent advances in metabolomics technologies. Here, we review techniques for measuring metabolic fluxes in intact mammals and discuss how to analyse and interpret the results. In tandem, we describe important findings from these techniques, and suggest promising avenues for their future application. Given the broad importance of metabolism to health and disease, more widespread application of these methods holds the potential to accelerate biomedical progress.
© 2021. Springer Nature Limited.
Conflict of interest statement
Competing interests
The authors declare no competing interests.
Figures


References
-
- Fenn JB, Mann M, Meng CK, Wong SF & Whitehouse CM Electrospray ionization–principles and practice. Mass Spectrom. Rev 9, 37–70 (1990).
-
- Tanaka K et al. Protein and polymer analyses up to m/z 100 000 by laser ionization time-of-flight mass spectrometry. Rapid Commun. Mass Spectrom 2, 151–153 (1988).
-
- Wolfe RR Tracers in Metabolic Research: Radioisotope and Stable Isotope/Mass Spectometry Methods (A.R. Liss, 1984). - PubMed
-
- McCabe BJ & Previs SF Using isotope tracers to study metabolism: application in mouse models. Metab. Eng 6, 25–35 (2004). - PubMed
-
- Fernández-García J, Altea-Manzano P, Pranzini E & Fendt S-M Stable isotopes for tracing mammalian-cell metabolism in vivo. Trends Biochem. Sci 45, 185–201 (2020). - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

