Microglial Implications in SARS-CoV-2 Infection and COVID-19: Lessons From Viral RNA Neurotropism and Possible Relevance to Parkinson's Disease
- PMID: 34211370
- PMCID: PMC8240959
- DOI: 10.3389/fncel.2021.670298
Microglial Implications in SARS-CoV-2 Infection and COVID-19: Lessons From Viral RNA Neurotropism and Possible Relevance to Parkinson's Disease
Abstract
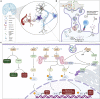
Since December 2019, humankind has been experiencing a ravaging severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) outbreak, the second coronavirus pandemic in a decade after the Middle East respiratory syndrome coronavirus (MERS-CoV) disease in 2012. Infection with SARS-CoV-2 results in Coronavirus disease 2019 (COVID-19), which is responsible for over 3.1 million deaths worldwide. With the emergence of a second and a third wave of infection across the globe, and the rising record of multiple reinfections and relapses, SARS-CoV-2 infection shows no sign of abating. In addition, it is now evident that SARS-CoV-2 infection presents with neurological symptoms that include early hyposmia, ischemic stroke, meningitis, delirium and falls, even after viral clearance. This may suggest chronic or permanent changes to the neurons, glial cells, and/or brain vasculature in response to SARS-CoV-2 infection or COVID-19. Within the central nervous system (CNS), microglia act as the central housekeepers against altered homeostatic states, including during viral neurotropic infections. In this review, we highlight microglial responses to viral neuroinfections, especially those with a similar genetic composition and route of entry as SARS-CoV-2. As the primary sensor of viral infection in the CNS, we describe the pathogenic and neuroinvasive mechanisms of RNA viruses and SARS-CoV-2 vis-à-vis the microglial means of viral recognition. Responses of microglia which may culminate in viral clearance or immunopathology are also covered. Lastly, we further discuss the implication of SARS-CoV-2 CNS invasion on microglial plasticity and associated long-term neurodegeneration. As such, this review provides insight into some of the mechanisms by which microglia could contribute to the pathophysiology of post-COVID-19 neurological sequelae and disorders, including Parkinson's disease, which could be pervasive in the coming years given the growing numbers of infected and re-infected individuals globally.
Keywords: COVID-19; Parkinson’s disease; SARS-CoV-2; brain; microglia; neurodegenerative diseases; neuropsychiatric disorders; viral RNA neurotropism.
Copyright © 2021 Awogbindin, Ben-Azu, Olusola, Akinluyi, Adeniyi, Di Paolo and Tremblay.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Adhikari S. P., Meng S., Wu Y. J., Mao Y. P., Ye R. X., Wang Q. Z., et al. (2020). Epidemiology, causes, clinical manifestation and diagnosis, prevention and control of coronavirus disease (COVID-19) during the early outbreak period: a scoping review. Infect. Dis. Poverty 9:29. 10.1186/s40249-020-00646-x - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

