Exclusion of the Possibility of "False Ripples" From Ripple Band High-Frequency Oscillations Recorded From Scalp Electroencephalogram in Children With Epilepsy
- PMID: 34211382
- PMCID: PMC8239160
- DOI: 10.3389/fnhum.2021.696882
Exclusion of the Possibility of "False Ripples" From Ripple Band High-Frequency Oscillations Recorded From Scalp Electroencephalogram in Children With Epilepsy
Abstract
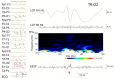
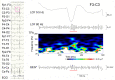
Aim: Ripple-band epileptic high-frequency oscillations (HFOs) can be recorded by scalp electroencephalography (EEG), and tend to be associated with epileptic spikes. However, there is a concern that the filtration of steep waveforms such as spikes may cause spurious oscillations or "false ripples." We excluded such possibility from at least some ripples by EEG differentiation, which, in theory, enhances high-frequency signals and does not generate spurious oscillations or ringing.
Methods: The subjects were 50 pediatric patients, and ten consecutive spikes during sleep were selected for each patient. Five hundred spike data segments were initially reviewed by two experienced electroencephalographers using consensus to identify the presence or absence of ripples in the ordinary filtered EEG and an associated spectral blob in time-frequency analysis (Session A). These EEG data were subjected to numerical differentiation (the second derivative was denoted as EEG″). The EEG″ trace of each spike data segment was shown to two other electroencephalographers who judged independently whether there were clear ripple oscillations or uncertain ripple oscillations or an absence of oscillations (Session B).
Results: In Session A, ripples were identified in 57 spike data segments (Group A-R), but not in the other 443 data segments (Group A-N). In Session B, both reviewers identified clear ripples (strict criterion) in 11 spike data segments, all of which were in Group A-R (p < 0.0001 by Fisher's exact test). When the extended criterion that included clear and/or uncertain ripples was used in Session B, both reviewers identified 25 spike data segments that fulfilled the criterion: 24 of these were in Group A-R (p < 0.0001).
Discussion: We have demonstrated that real ripples over scalp spikes exist in a certain proportion of patients. Ripples that were visualized consistently using both ordinary filters and the EEG″ method should be true, but failure to clarify ripples using the EEG″ method does not mean that true ripples are absent.
Conclusion: The numerical differentiation of EEG data provides convincing evidence that HFOs were detected in terms of the presence of such unusually fast oscillations over the scalp and the importance of this electrophysiological phenomenon.
Keywords: child; epilepsy; false ripple; fast oscillation (FO); high-frequency oscillation (HFO); scalp EEG.
Copyright © 2021 Kobayashi, Shibata, Tsuchiya and Akiyama.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Scalp recorded spike ripples predict seizure risk in childhood epilepsy better than spikes.Brain. 2019 May 1;142(5):1296-1309. doi: 10.1093/brain/awz059. Brain. 2019. PMID: 30907404 Free PMC article.
-
High Frequency Oscillations in the Ripple Band (80-250 Hz) in Scalp EEG: Higher Density of Electrodes Allows for Better Localization of the Seizure Onset Zone.Brain Topogr. 2018 Nov;31(6):1059-1072. doi: 10.1007/s10548-018-0658-3. Epub 2018 Jul 6. Brain Topogr. 2018. PMID: 29980967
-
Physiological Ripples Associated With Sleep Spindles Can Be Identified in Patients With Refractory Epilepsy Beyond Mesio-Temporal Structures.Front Neurol. 2021 Feb 10;12:612293. doi: 10.3389/fneur.2021.612293. eCollection 2021. Front Neurol. 2021. PMID: 33643198 Free PMC article.
-
Epileptic High-frequency Oscillations in Scalp Electroencephalography.Acta Med Okayama. 2018 Aug;72(4):325-329. doi: 10.18926/AMO/56166. Acta Med Okayama. 2018. PMID: 30140078 Review.
-
High-frequency oscillations in scalp EEG: A systematic review of methodological choices and clinical findings.Clin Neurophysiol. 2022 May;137:46-58. doi: 10.1016/j.clinph.2021.12.017. Epub 2022 Jan 20. Clin Neurophysiol. 2022. PMID: 35272185
Cited by
-
Scalp HFO rates are higher for larger lesions.Epilepsia Open. 2022 Sep;7(3):496-503. doi: 10.1002/epi4.12596. Epub 2022 May 6. Epilepsia Open. 2022. PMID: 35357778 Free PMC article.
-
Phase slips extracted from derivatives of EEG data provide a deeper insight into the formation of cortical phase transitions.Front Integr Neurosci. 2025 Feb 25;19:1471120. doi: 10.3389/fnint.2025.1471120. eCollection 2025. Front Integr Neurosci. 2025. PMID: 40070796 Free PMC article.
-
Implementation of a Morphological Filter for Removing Spikes from the Epileptic Brain Signals to Improve Identification Ripples.Sensors (Basel). 2022 Oct 4;22(19):7522. doi: 10.3390/s22197522. Sensors (Basel). 2022. PMID: 36236621 Free PMC article.
References
-
- Bernardo D., Nariai H., Hussain S. A., Sankar R., Salamon N., Krueger D. A., et al. (2018). Visual and semi-automatic non-invasive detection of interictal fast ripples: a potential biomarker of epilepsy in children with tuberous sclerosis complex. Clin. Neurophysiol. 129 1458–1466. 10.1016/j.clinph.2018.03.010 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

