Thyroid Function in Preterm/Low Birth Weight Infants: Impact on Diagnosis and Management of Thyroid Dysfunction
- PMID: 34211436
- PMCID: PMC8239410
- DOI: 10.3389/fendo.2021.666207
Thyroid Function in Preterm/Low Birth Weight Infants: Impact on Diagnosis and Management of Thyroid Dysfunction
Abstract
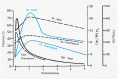
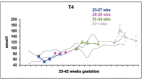
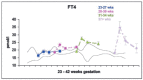
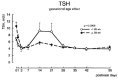
Maternal thyroid hormone crosses the placenta to the fetus beginning in the first trimester, likely playing an important role in fetal development. The fetal thyroid gland begins to produce thyroid hormone in the second trimester, with fetal serum T4 levels gradually rising to term. Full maturation of the hypothalamic-pituitary-thyroid (HPT) axis does not occur until term gestation or the early neonatal period. Postnatal thyroid function in preterm babies is qualitatively similar to term infants, but the TSH surge is reduced, with a corresponding decrease in the rise in T4 and T3 levels. Serum T4 levels are reduced in proportion to the degree of prematurity, representing both loss of the maternal contribution and immaturity of the HPT axis. Other factors, such as neonatal drugs, e.g., dopamine, and non-thyroidal illness syndrome (NTIS) related to co-morbidities contribute to the "hypothyroxinemia of prematurity". Iodine, both deficiency and excess, may impact thyroid function in infants born preterm. Overall, the incidence of permanent congenital hypothyroidism in preterm infants appears to be similar to term infants. However, in newborn screening (NBS) that employ a total T4-reflex TSH test approach, a higher proportion of preterm babies will have a T4 below the cutoff, associated with a non-elevated TSH level. In NBS programs with a primary TSH test combined with serial testing, there is a relatively high incidence of "delayed TSH elevation" in preterm neonates. On follow-up, the majority of these cases have transient hypothyroidism. Preterm/LBW infants have many clinical manifestations that might be ascribed to hypothyroidism. The question then arises whether the hypothyroxinemia of prematurity, with thyroid function tests compatible with either non-thyroidal illness syndrome or central hypothyroidism, is a physiologic or pathologic process. In particular, does hypothyroxinemia contribute to the neurodevelopmental impairment common to preterm infants? Results from multiple studies are mixed, with some randomized controlled trials in the most preterm infants born <28 weeks gestation appearing to show benefit. This review will summarize fetal and neonatal thyroid physiology, thyroid disorders specific to preterm/LBW infants and their impact on NBS for congenital hypothyroidism, examine treatment studies, and finish with comments on unresolved questions and areas of controversy.
Keywords: congenital hypothyroidism; iodine; low birth weight; newborn screening; preterm; thyroid function.
Copyright © 2021 LaFranchi.
Conflict of interest statement
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

