Potential Utilization of APOBEC3-Mediated Mutagenesis for an HIV-1 Functional Cure
- PMID: 34211449
- PMCID: PMC8239295
- DOI: 10.3389/fmicb.2021.686357
Potential Utilization of APOBEC3-Mediated Mutagenesis for an HIV-1 Functional Cure
Abstract
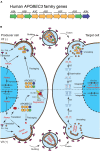
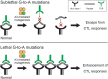
The introduction of combination antiretroviral therapy (cART) has managed to control the replication of human immunodeficiency virus type 1 (HIV-1) in infected patients. However, a complete HIV-1 cure, including a functional cure for or eradication of HIV-1, has yet to be achieved because of the persistence of latent HIV-1 reservoirs in adherent patients. The primary source of these viral reservoirs is integrated proviral DNA in CD4+ T cells and other non-T cells. Although a small fraction of this proviral DNA is replication-competent and contributes to viral rebound after the cessation of cART, >90% of latent viral reservoirs are replication-defective and some contain high rates of G-to-A mutations in proviral DNA. At least in part, these high rates of G-to-A mutations arise from the APOBEC3 (A3) family proteins of cytosine deaminases. A general model has shown that the HIV-1 virus infectivity factor (Vif) degrades A3 family proteins by proteasome-mediated pathways and inactivates their antiviral activities. However, Vif does not fully counteract the HIV-1 restriction activity of A3 family proteins in vivo, as indicated by observations of A3-mediated G-to-A hypermutation in the proviral DNA of HIV-1-infected patients. The frequency of A3-mediated hypermutation potentially contributes to slower HIV-1/AIDS disease progression and virus evolution including the emergence of cytotoxic T lymphocyte escape mutants. Therefore, combined with other strategies, the manipulation of A3-mediated mutagenesis may contribute to an HIV-1 functional cure aimed at cART-free remission. In this mini-review, we discuss the possibility of an HIV-1 functional cure arising from manipulation of A3 mutagenic activity.
Keywords: A3 expression; A3-interacting proteins; APOBEC3-mediated mutagenesis; Vif inhibitors; adaptive immunity; genetic factors.
Copyright © 2021 Ikeda, Yue, Shimizu and Nasser.
Conflict of interest statement
The reviewer DS declared a past co-authorship with the author TI to the handling editor. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
APOBEC3 degradation is the primary function of HIV-1 Vif for virus replication in the myeloid cell line THP-1.bioRxiv [Preprint]. 2023 Mar 29:2023.03.28.534666. doi: 10.1101/2023.03.28.534666. bioRxiv. 2023. Update in: mBio. 2023 Aug 31;14(4):e0078223. doi: 10.1128/mbio.00782-23. PMID: 37034786 Free PMC article. Updated. Preprint.
-
Structural perspectives on HIV-1 Vif and APOBEC3 restriction factor interactions.Protein Sci. 2020 Feb;29(2):391-406. doi: 10.1002/pro.3729. Epub 2019 Nov 29. Protein Sci. 2020. PMID: 31518043 Free PMC article. Review.
-
A New Class of Antiretroviral Enabling Innate Immunity by Protecting APOBEC3 from HIV Vif-Dependent Degradation.Trends Mol Med. 2018 May;24(5):507-520. doi: 10.1016/j.molmed.2018.03.004. Epub 2018 Mar 30. Trends Mol Med. 2018. PMID: 29609878 Free PMC article. Review.
-
A Naturally Occurring Domestic Cat APOBEC3 Variant Confers Resistance to Feline Immunodeficiency Virus Infection.J Virol. 2015 Oct 21;90(1):474-85. doi: 10.1128/JVI.02612-15. Print 2016 Jan 1. J Virol. 2015. PMID: 26491161 Free PMC article.
-
APOBEC3-mediated editing in HIV type 1 from pediatric patients and its association with APOBEC3G/CUL5 polymorphisms and Vif variability.AIDS Res Hum Retroviruses. 2012 Jun;28(6):619-27. doi: 10.1089/AID.2011.0291. Epub 2012 Feb 2. AIDS Res Hum Retroviruses. 2012. PMID: 22145963
Cited by
-
APOBEC3 degradation is the primary function of HIV-1 Vif for virus replication in the myeloid cell line THP-1.bioRxiv [Preprint]. 2023 Mar 29:2023.03.28.534666. doi: 10.1101/2023.03.28.534666. bioRxiv. 2023. Update in: mBio. 2023 Aug 31;14(4):e0078223. doi: 10.1128/mbio.00782-23. PMID: 37034786 Free PMC article. Updated. Preprint.
-
Potential Role of APOBEC3 Family Proteins in SARS-CoV-2 Replication.Viruses. 2024 Jul 16;16(7):1141. doi: 10.3390/v16071141. Viruses. 2024. PMID: 39066304 Free PMC article.
-
Genomic instability genes in lung and colon adenocarcinoma indicate organ specificity of transcriptomic impact on Copy Number Alterations.Sci Rep. 2022 Jul 11;12(1):11739. doi: 10.1038/s41598-022-15692-8. Sci Rep. 2022. PMID: 35817785 Free PMC article.
-
Potent dual block to HIV-1 infection using lentiviral vectors expressing fusion inhibitor peptide mC46- and Vif-resistant APOBEC3G.Mol Ther Nucleic Acids. 2023 Aug 11;33:794-809. doi: 10.1016/j.omtn.2023.08.007. eCollection 2023 Sep 12. Mol Ther Nucleic Acids. 2023. PMID: 37662965 Free PMC article.
-
Design of Vif-Derived Peptide Inhibitors with Anti-HIV-1 Activity by Interrupting Vif-CBFβ Interaction.Viruses. 2024 Mar 22;16(4):490. doi: 10.3390/v16040490. Viruses. 2024. PMID: 38675833 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

