Outstanding Response in a Patient With ROS1-Rearranged Inflammatory Myofibroblastic Tumor of Soft Tissues Treated With Crizotinib: Case Report
- PMID: 34211840
- PMCID: PMC8239351
- DOI: 10.3389/fonc.2021.658327
Outstanding Response in a Patient With ROS1-Rearranged Inflammatory Myofibroblastic Tumor of Soft Tissues Treated With Crizotinib: Case Report
Abstract
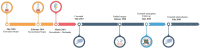
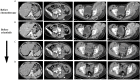
Inflammatory myofibroblastic tumor (IMT) is a very rare subtype of sarcoma, which frequently harbor chromosomal rearrangements, including anaplastic lymphoma kinase (ALK) rearrangements (almost 50% of the IMTs) and other kinase fusions such as ROS1. ROS1 fusions are present in about 10% of IMT, almost half of the ALK-negative IMT patients. Apart from radical surgery for resectable tumors, there is no standard-of-care therapy for advanced IMTs. Nonetheless, the use of tyrosine kinase inhibitors has shown promising efficacy in IMT patients with targetable genomic alterations. We report the case of a 24-year-old patient with chemotherapy-refractory metastatic IMT harboring ROS1 kinase fusion, who experienced a significant clinical and pathological response to crizotinib. This clinical case highlights the need to assess all patients with unresectable IMTs for chromosomal abnormalities and gene mutations and address them to targeted agents as well as clinical trials.
Keywords: ROS1; crizotinib; inflammatory myofibroblastic tumor; inflammatory pseudotumor; retreatment; sarcoma; target therapy.
Copyright © 2021 Comandini, Catalano, Grassi, Pesola, Bertulli, Guadagno, Spina, Mascherini, De Cian, Pistoia and Rebuzzi.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
Publication types
LinkOut - more resources
Full Text Sources

