Omics Approaches to Study Formation and Function of Human Placental Syncytiotrophoblast
- PMID: 34211975
- PMCID: PMC8240757
- DOI: 10.3389/fcell.2021.674162
Omics Approaches to Study Formation and Function of Human Placental Syncytiotrophoblast
Abstract
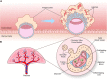
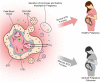
Proper development of the placenta is vital for pregnancy success. The placenta regulates exchange of nutrients and gases between maternal and fetal blood and produces hormones essential to maintain pregnancy. The placental cell lineage primarily responsible for performing these functions is a multinucleated entity called syncytiotrophoblast. Syncytiotrophoblast is continuously replenished throughout pregnancy by fusion of underlying progenitor cells called cytotrophoblasts. Dysregulated syncytiotrophoblast formation disrupts the integrity of the placental exchange surface, which can be detrimental to maternal and fetal health. Moreover, various factors produced by syncytiotrophoblast enter into maternal circulation, where they profoundly impact maternal physiology and are promising diagnostic indicators of pregnancy health. Despite the multifunctional importance of syncytiotrophoblast for pregnancy success, there is still much to learn about how its formation is regulated in normal and diseased states. 'Omics' approaches are gaining traction in many fields to provide a more holistic perspective of cell, tissue, and organ function. Herein, we review human syncytiotrophoblast development and current model systems used for its study, discuss how 'omics' strategies have been used to provide multidimensional insights into its formation and function, and highlight limitations of current platforms as well as consider future avenues for exploration.
Keywords: cell models; omics; placenta; pregnancy; syncytiotrophoblast; trophoblast.
Copyright © 2021 Jaremek, Jeyarajah, Jaju Bhattad and Renaud.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Azar C., Valentine M., Trausch-Azar J., Druley T., Nelson D. M., Schwartz A. L. (2018). RNA-Seq identifies genes whose proteins are transformative in the differentiation of cytotrophoblast to syncytiotrophoblast, in human primary villous and BeWo trophoblasts. Sci. Rep. 8:5142. 10.1038/s41598-018-23379-2 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

