Lineage Differentiation Markers as a Proxy for Embryo Viability in Farm Ungulates
- PMID: 34212020
- PMCID: PMC8239129
- DOI: 10.3389/fvets.2021.680539
Lineage Differentiation Markers as a Proxy for Embryo Viability in Farm Ungulates
Abstract
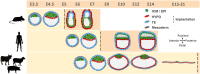
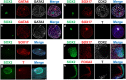
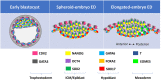
Embryonic losses constitute a major burden for reproductive efficiency of farm animals. Pregnancy losses in ungulate species, which include cattle, pigs, sheep and goats, majorly occur during the second week of gestation, when the embryo experiences a series of cell differentiation, proliferation, and migration processes encompassed under the term conceptus elongation. Conceptus elongation takes place following blastocyst hatching and involves a massive proliferation of the extraembryonic membranes trophoblast and hypoblast, and the formation of flat embryonic disc derived from the epiblast, which ultimately gastrulates generating the three germ layers. This process occurs prior to implantation and it is exclusive from ungulates, as embryos from other mammalian species such as rodents or humans implant right after hatching. The critical differences in embryo development between ungulates and mice, the most studied mammalian model, have precluded the identification of the genes governing lineage differentiation in livestock species. Furthermore, conceptus elongation has not been recapitulated in vitro, hindering the study of these cellular events. Luckily, recent advances on transcriptomics, genome modification and post-hatching in vitro culture are shedding light into this largely unknown developmental window, uncovering possible molecular markers to determine embryo quality. In this review, we summarize the events occurring during ungulate pre-implantation development, highlighting recent findings which reveal that several dogmas in Developmental Biology established by knock-out murine models do not hold true for other mammals, including humans and farm animals. The developmental failures associated to in vitro produced embryos in farm animals are also discussed together with Developmental Biology tools to assess embryo quality, including molecular markers to assess proper lineage commitment and a post-hatching in vitro culture system able to directly determine developmental potential circumventing the need of experimental animals.
Keywords: assisted reproductive technologies; conceptus elongation; developmental biology; embryo quality; embryo transfer; lineage markers; post-hatching embryo culture; viability.
Copyright © 2021 Pérez-Gómez, González-Brusi, Bermejo-Álvarez and Ramos-Ibeas.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
In vitro culture of ovine embryos up to early gastrulating stages.Development. 2022 Mar 15;149(6):dev199743. doi: 10.1242/dev.199743. Epub 2022 Mar 23. Development. 2022. PMID: 35319748 Free PMC article.
-
Embryonic disc formation following post-hatching bovine embryo development in vitro.Reproduction. 2020 Oct;160(4):579-589. doi: 10.1530/REP-20-0243. Reproduction. 2020. PMID: 32698149 Free PMC article.
-
Pre-hatching exposure to N2B27 medium improves post-hatching development of bovine embryos in vitro.Theriogenology. 2023 Jul 15;205:73-78. doi: 10.1016/j.theriogenology.2023.04.018. Epub 2023 Apr 18. Theriogenology. 2023. PMID: 37087966
-
Blastocyst elongation, trophoblastic differentiation, and embryonic pattern formation.Reproduction. 2008 Feb;135(2):181-95. doi: 10.1530/REP-07-0355. Reproduction. 2008. PMID: 18239048 Review.
-
Post-hatching development of the porcine and bovine embryo--defining criteria for expected development in vivo and in vitro.Theriogenology. 2006 Jan 7;65(1):153-65. doi: 10.1016/j.theriogenology.2005.09.021. Epub 2005 Oct 28. Theriogenology. 2006. PMID: 16257443 Review.
Cited by
-
PPARG is dispensable for bovine embryo development up to tubular stages†.Biol Reprod. 2024 Sep 14;111(3):557-566. doi: 10.1093/biolre/ioae083. Biol Reprod. 2024. PMID: 38832705 Free PMC article.
-
In Vitro-Produced Equine Blastocysts Exhibit Greater Dispersal and Intermingling of Inner Cell Mass Cells than In Vivo Embryos.Int J Mol Sci. 2023 Jun 1;24(11):9619. doi: 10.3390/ijms24119619. Int J Mol Sci. 2023. PMID: 37298570 Free PMC article.
-
Defining core signaling pathways for supporting in vitro maintenance of pig extraembryonic endoderm (XEN) cells.Reproduction. 2025 Mar 11;169(4):e240393. doi: 10.1530/REP-24-0393. Print 2025 Apr 1. Reproduction. 2025. PMID: 40019752 Free PMC article.
-
SMC2 ablation impairs bovine embryo development shortly after blastocyst hatching.Reproduction. 2024 Oct 3;168(5):e240211. doi: 10.1530/REP-24-0211. Print 2024 Nov 1. Reproduction. 2024. PMID: 39231091 Free PMC article.
-
Understanding bovine embryo elongation: a transcriptomic study of trophoblastic vesicles.Front Physiol. 2024 Jan 29;15:1331098. doi: 10.3389/fphys.2024.1331098. eCollection 2024. Front Physiol. 2024. PMID: 38348224 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources

