Evaluation of Antigenic Comparisons Among BVDV Isolates as it Relates to Humoral and Cell Mediated Responses
- PMID: 34212022
- PMCID: PMC8239304
- DOI: 10.3389/fvets.2021.685114
Evaluation of Antigenic Comparisons Among BVDV Isolates as it Relates to Humoral and Cell Mediated Responses
Abstract
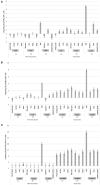
Antigenic differences between bovine viral diarrhea virus (BVDV) vaccine strains and field isolates can lead to reduced vaccine efficacy. Historically, antigenic differences among BVDV strains were evaluated using techniques based on polyclonal and monoclonal antibody activity. The most common method for antigenic comparison among BVDV isolates is determination of virus neutralization titer (VNT). BVDV antigenic comparisons using VNT only account for the humoral component of the adaptive immune response, and not cell mediated immunity (CMI) giving an incomplete picture of protective responses. Currently, little data is available regarding potential antigenic differences between BVDV vaccine strains and field isolates as measured by CMI responses. The goal of the current paper is to evaluate two groups of cattle that differed in the frequency they were vaccinated, to determine if similar trends in CMI responses exist within each respective group when stimulated with antigenically different BVDV strains. Data from the current study demonstrated variability in the CMI response is associated with the viral strain used for stimulation. Variability in IFN-γ mRNA expression was most pronounced in the CD4+ population, this was observed between the viruses within each respective BVDV subgenotype in the Group 1 calves. The increase in frequency of CD25+ cells and IFN-γ mRNA expression in the CD8+ and CD335+ populations were not as variable between BVDV strains used for stimulation in the Group 1 calves. Additionally, an inverse relationship between VNT and IFN-γ mRNA expression was observed, as the lowest VNT and highest IFN-γ mRNA expression was observed and vice versa, the highest VNT and lowest IFN-γ mRNA expression was observed. A similar trend regardless of vaccination status was observed between the two groups of calves, as the BVDV-1b strain had lower IFN-γ mRNA expression. Collectively, data from the current study and previous data support, conferring protection against BVDV as a method for control of BVDV in cattle populations is still a complex issue and requires a multifactorial approach to understand factors associated with vaccine efficacy or conversely vaccine failure. Although, there does appear to be an antigenic component associated with CMI responses as well as with humoral responses as determined by VNT.
Keywords: antigenic diversity; antigenicity; bovine viral diarrhea virus; cell mediated response; virus neutralizing titer.
Copyright © 2021 Falkenberg, Dassanayake, Terhaar, Ridpath, Neill and Roth.
Conflict of interest statement
JFR was employed by company Ridpath Consulting, LLC. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Multivariate Analysis as a Method to Evaluate Antigenic Relationships between Bovine Viral Diarrhea Virus 1b Isolates and Vaccine Strains.Viruses. 2023 Oct 13;15(10):2085. doi: 10.3390/v15102085. Viruses. 2023. PMID: 37896862 Free PMC article.
-
Measuring CMI responses using the PrimeFlow RNA assay: A new method of evaluating BVDV vaccination response in cattle.Vet Immunol Immunopathol. 2020 Mar;221:110024. doi: 10.1016/j.vetimm.2020.110024. Epub 2020 Feb 11. Vet Immunol Immunopathol. 2020. PMID: 32070831
-
Multivariate analysis reveals that BVDV field isolates do not show a close VN-based antigenic relationship to US vaccine strains.BMC Res Notes. 2023 Jun 26;16(1):121. doi: 10.1186/s13104-023-06410-2. BMC Res Notes. 2023. PMID: 37365644 Free PMC article.
-
Impact of species and subgenotypes of bovine viral diarrhea virus on control by vaccination.Anim Health Res Rev. 2015 Jun;16(1):40-54. doi: 10.1017/S1466252315000079. Anim Health Res Rev. 2015. PMID: 26050571 Review.
-
Bovine viral diarrhea virus proteins and their antigenic analyses.Arch Virol Suppl. 1991;3:29-40. doi: 10.1007/978-3-7091-9153-8_4. Arch Virol Suppl. 1991. PMID: 9210923 Review.
Cited by
-
Response to Bovine Viral Diarrhea Virus in Heifers Vaccinated with a Combination of Multivalent Modified Live and Inactivated Viral Vaccines.Viruses. 2023 Mar 8;15(3):703. doi: 10.3390/v15030703. Viruses. 2023. PMID: 36992412 Free PMC article.
-
Multivariate Analysis as a Method to Evaluate Antigenic Relationships between Bovine Viral Diarrhea Virus 1b Isolates and Vaccine Strains.Viruses. 2023 Oct 13;15(10):2085. doi: 10.3390/v15102085. Viruses. 2023. PMID: 37896862 Free PMC article.
-
Efficacy of Vaccination with the DIVENCE® Vaccine Against Bovine Viral Diarrhea Virus Types 1 and 2 in Terms of Fetal Protection.Vet Med (Auckl). 2024 Dec 10;15:221-238. doi: 10.2147/VMRR.S474655. eCollection 2024. Vet Med (Auckl). 2024. PMID: 39679301 Free PMC article.
-
Classical swine fever: Unveiling the complexity through a multifaceted approach.Open Vet J. 2024 Oct;14(10):2497-2508. doi: 10.5455/OVJ.2024.v14.i10.1. Epub 2024 Oct 31. Open Vet J. 2024. PMID: 39545196 Free PMC article. Review.
-
Characterization of Cellular and Humoral Immunity to Commercial Cattle BVDV Vaccines in White-Tailed Deer.Vaccines (Basel). 2025 Apr 18;13(4):427. doi: 10.3390/vaccines13040427. Vaccines (Basel). 2025. PMID: 40333321 Free PMC article.
References
-
- Deregt D. Introduction and history. In: Ridpath J, Goyal S, editors. Bovine Viral Diarrhea Virus: Diagnosis, Management, and Control. Hoboken, NJ: Blackwell Publishing; (2005). p. 3–33.
LinkOut - more resources
Full Text Sources
Research Materials

