Vibrio fischeri imports and assimilates sulfate during symbiosis with Euprymna scolopes
- PMID: 34212439
- PMCID: PMC8514163
- DOI: 10.1111/mmi.14780
Vibrio fischeri imports and assimilates sulfate during symbiosis with Euprymna scolopes
Abstract
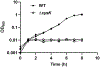
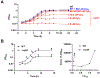
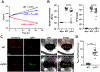
Sulfur is in cellular components of bacteria and is, therefore, an element necessary for growth. However, mechanisms by which bacteria satisfy their sulfur needs within a host are poorly understood. Vibrio fischeri is a bacterial symbiont that colonizes, grows, and produces bioluminescence within the light organ of the Hawaiian bobtail squid, which provides an experimental platform for investigating sulfur acquisition in vivo. Like other γ-proteobacteria, V. fischeri fuels sulfur-dependent anabolic processes with intracellular cysteine. Within the light organ, the abundance of a ΔcysK mutant, which cannot synthesize cysteine through sulfate assimilation, is attenuated, suggesting sulfate import is necessary for V. fischeri to establish symbiosis. Genes encoding sulfate-import systems of other bacteria that assimilate sulfate were not identified in the V. fischeri genome. A transposon mutagenesis screen implicated YfbS as a sulfate importer. YfbS is necessary for growth on sulfate and in the marine environment. During symbiosis, a ΔyfbS mutant is attenuated and strongly expresses sulfate-assimilation genes, which is a phenotype associated with sulfur-starved cells. Together, these results suggest V. fischeri imports sulfate via YfbS within the squid light organ, which provides insight into the molecular mechanisms by which bacteria harvest sulfur in vivo.
Keywords: Vibrio; host-microbe interactions; sulfate assimilation; symbiosis; transport.
© 2021 John Wiley & Sons Ltd.
Conflict of interest statement
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Figures
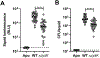




References
-
- Assur Sanghai Z, Liu Q, Clarke OB, Belcher-Dufrisne M, Wiriyasermkul P, Giese MH, Leal-Pinto E, Kloss B, Tabuso S, Love J, Punta M, Banerjee S, Rajashankar KR, Rost B, Logothetis D, Quick M, Hendrickson WA and Mancia F. (2018) Structure-based analysis of CysZ-mediated cellular uptake of sulfate. Elife, 7, e27829. 10.7554/eLife.27829 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

