Neutralising capacity against Delta (B.1.617.2) and other variants of concern following Comirnaty (BNT162b2, BioNTech/Pfizer) vaccination in health care workers, Israel
- PMID: 34212838
- PMCID: PMC8326656
- DOI: 10.2807/1560-7917.ES.2021.26.26.2100557
Neutralising capacity against Delta (B.1.617.2) and other variants of concern following Comirnaty (BNT162b2, BioNTech/Pfizer) vaccination in health care workers, Israel
Abstract
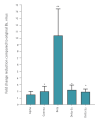
SARS-CoV-2 Delta (B.1.617.2) variant of concern (VOC) and other VOCs are spreading in Europe. Micro-neutralisation assays with sera obtained after Comirnaty (BNT162b2, BioNTech/Pfizer) vaccination in 36 healthcare workers (31 female) demonstrated significant fold change reduction in neutralising titres compared with the original virus: Gamma (P.1) 2.3, Beta (B.1.351) 10.4, Delta 2.1 and 2.6. The reduction of the Alpha (B.1.1.7) variant was not significant. Despite being lower, remaining neutralisation capacity conferred by Comirnaty against Delta and other VOCs is probably protective.
Keywords: B.1.617.2 (Delta); BNT162b2 vaccination; COVID-19; neutralizing; variants.
Conflict of interest statement
Figures


References
-
- Haas EJ, Angulo FJ, McLaughlin JM, Anis E, Singer SR, Khan F, et al. Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: an observational study using national surveillance data. Lancet. 2021;397(10287):1819-29. 10.1016/S0140-6736(21)00947-8 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
