A novel mechanosensitive channel controls osmoregulation, differentiation, and infectivity in Trypanosoma cruzi
- PMID: 34212856
- PMCID: PMC8282336
- DOI: 10.7554/eLife.67449
A novel mechanosensitive channel controls osmoregulation, differentiation, and infectivity in Trypanosoma cruzi
Abstract
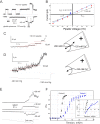
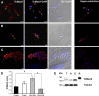
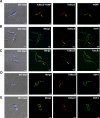
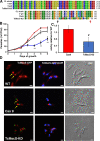
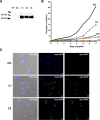
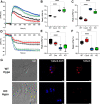
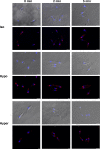
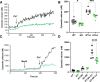
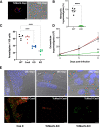
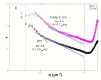
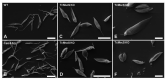
The causative agent of Chagas disease undergoes drastic morphological and biochemical modifications as it passes between hosts and transitions from extracellular to intracellular stages. The osmotic and mechanical aspects of these cellular transformations are not understood. Here we identify and characterize a novel mechanosensitive channel in Trypanosoma cruzi (TcMscS) belonging to the superfamily of small-conductance mechanosensitive channels (MscS). TcMscS is activated by membrane tension and forms a large pore permeable to anions, cations, and small osmolytes. The channel changes its location from the contractile vacuole complex in epimastigotes to the plasma membrane as the parasites develop into intracellular amastigotes. TcMscS knockout parasites show significant fitness defects, including increased cell volume, calcium dysregulation, impaired differentiation, and a dramatic decrease in infectivity. Our work provides mechanistic insights into components supporting pathogen adaptation inside the host, thus opening the exploration of mechanosensation as a prerequisite for protozoan infectivity.
Keywords: CRISPR-Cas9; Trypanosoma cruzi; calcium; electrophysiology; infectious disease; mechanosensation; microbiology; osmoregulation.
© 2021, Dave et al.
Conflict of interest statement
ND, UC, DA, JF, MT, PB, NL, AA, SS, VJ No competing interests declared
Figures







References
-
- Aistleitner K, Heinz C, Hörmann A, Heinz E, Montanaro J, Schulz F, Maier E, Pichler P, Benz R, Horn M. Identification and characterization of a novel porin family highlights a major difference in the outer membrane of chlamydial symbionts and pathogens. PLOS ONE. 2013;8:e55010. doi: 10.1371/journal.pone.0055010. - DOI - PMC - PubMed
-
- Andersen MN, Hefting LL, Steffensen AB, Schmitt N, Olesen SP, Olsen JV, Lundby A, Rasmussen HB. Protein kinase A stimulates Kv7.1 surface expression by regulating Nedd4-2-dependent endocytic trafficking. American Journal of Physiology-Cell Physiology. 2015;309:C693–C706. doi: 10.1152/ajpcell.00383.2014. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

