Frequency of Thrombocytopenia and Platelet Factor 4/Heparin Antibodies in Patients With Cerebral Venous Sinus Thrombosis Prior to the COVID-19 Pandemic
- PMID: 34213527
- PMCID: PMC8317004
- DOI: 10.1001/jama.2021.9889
Frequency of Thrombocytopenia and Platelet Factor 4/Heparin Antibodies in Patients With Cerebral Venous Sinus Thrombosis Prior to the COVID-19 Pandemic
Abstract
Importance: Cases of cerebral venous sinus thrombosis in combination with thrombocytopenia have recently been reported within 4 to 28 days of vaccination with the ChAdOx1 nCov-19 (AstraZeneca/Oxford) and Ad.26.COV2.S (Janssen/Johnson & Johnson) COVID-19 vaccines. An immune-mediated response associated with platelet factor 4/heparin antibodies has been proposed as the underlying pathomechanism.
Objective: To determine the frequencies of admission thrombocytopenia, heparin-induced thrombocytopenia, and presence of platelet factor 4/heparin antibodies in patients diagnosed with cerebral venous sinus thrombosis prior to the COVID-19 pandemic.
Design, setting, and participants: This was a descriptive analysis of a retrospective sample of consecutive patients diagnosed with cerebral venous sinus thrombosis between January 1987 and March 2018 from 7 hospitals participating in the International Cerebral Venous Sinus Thrombosis Consortium from Finland, the Netherlands, Switzerland, Sweden, Mexico, Iran, and Costa Rica. Of 952 patients, 865 with available baseline platelet count were included. In a subset of 93 patients, frozen plasma samples collected during a previous study between September 2009 and February 2016 were analyzed for the presence of platelet factor 4/heparin antibodies.
Exposures: Diagnosis of cerebral venous sinus thrombosis.
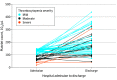
Main outcomes and measures: Frequencies of admission thrombocytopenia (platelet count <150 ×103/μL), heparin-induced thrombocytopenia (as diagnosed by the treating physician), and platelet factor 4/heparin IgG antibodies (optical density >0.4, in a subset of patients with previously collected plasma samples).
Results: Of 865 patients (median age, 40 years [interquartile range, 29-53 years], 70% women), 73 (8.4%; 95% CI, 6.8%-10.5%) had thrombocytopenia, which was mild (100-149 ×103/μL) in 52 (6.0%), moderate (50-99 ×103/μL) in 17 (2.0%), and severe (<50 ×103/μL) in 4 (0.5%). Heparin-induced thrombocytopenia with platelet factor 4/heparin antibodies was diagnosed in a single patient (0.1%; 95% CI, <0.1%-0.7%). Of the convenience sample of 93 patients with cerebral venous sinus thrombosis included in the laboratory analysis, 8 (9%) had thrombocytopenia, and none (95% CI, 0%-4%) had platelet factor 4/heparin antibodies.
Conclusions and relevance: In patients with cerebral venous sinus thrombosis prior to the COVID-19 pandemic, baseline thrombocytopenia was uncommon, and heparin-induced thrombocytopenia and platelet factor 4/heparin antibodies were rare. These findings may inform investigations of the possible association between the ChAdOx1 nCoV-19 and Ad26.COV2.S COVID-19 vaccines and cerebral venous sinus thrombosis with thrombocytopenia.
Conflict of interest statement
Figures

References
-
- European Medicines Agency . Assessment report: procedure under Article 5(3) of regulation (EC) No 726/2004—vaxzevira. Accessed May 5, 2021. https://www.ema.europa.eu/en/documents/referral/use-vaxzevria-prevent-co...
-
- MacNeil JR, Su JR, Broder KR, et al. . Updated recommendations from the Advisory Committee on Immunization Practices for use of the Janssen (Johnson & Johnson) COVID-19 vaccine after reports of thrombosis with thrombocytopenia syndrome among vaccine recipients—United States, April 2021. MMWR Morb Mortal Wkly Rep. 2021;70(17):651-656. doi:10.15585/mmwr.mm7017e4 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

