The HSPG syndecan is a core organizer of cholinergic synapses
- PMID: 34213535
- PMCID: PMC8258370
- DOI: 10.1083/jcb.202011144
The HSPG syndecan is a core organizer of cholinergic synapses
Abstract
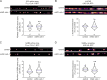
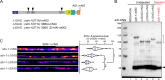
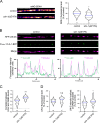
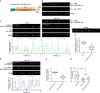
The extracellular matrix has emerged as an active component of chemical synapses regulating synaptic formation, maintenance, and homeostasis. The heparan sulfate proteoglycan (HSPG) syndecans are known to regulate cellular and axonal migration in the brain. They are also enriched at synapses, but their synaptic functions remain more elusive. Here, we show that SDN-1, the sole orthologue of syndecan in C. elegans, is absolutely required for the synaptic clustering of homomeric α7-like acetylcholine receptors (AChRs) and regulates the synaptic content of heteromeric AChRs. SDN-1 is concentrated at neuromuscular junctions (NMJs) by the neurally secreted synaptic organizer Ce-Punctin/MADD-4, which also activates the transmembrane netrin receptor DCC. Those cooperatively recruit the FARP and CASK orthologues that localize α7-like-AChRs at cholinergic NMJs through physical interactions. Therefore, SDN-1 stands at the core of the cholinergic synapse organization by bridging the extracellular synaptic determinants to the intracellular synaptic scaffold that controls the postsynaptic receptor content.
© 2021 Zhou et al.
Figures










References
-
- Alkondon, M., Pereira E.F., Barbosa C.T., and Albuquerque E.X.. 1997. Neuronal nicotinic acetylcholine receptor activation modulates gamma-aminobutyric acid release from CA1 neurons of rat hippocampal slices. J. Pharmacol. Exp. Ther. 283:1396–1411. - PubMed
-
- Altun, Z.F., Crocker C., and Hall D.H. 2021. Handbook of C. Elegans Anatomy. In WormAtlas.

